The mobility of the human adenosine A1 receptor-GFP fusion protein is dependent on receptor density at the surface of living CHO cells The human adenosine A1 receptor can couple to different G proteins in an agonist and receptor density-dependent manner (Cordeaux et al., 2000). Density-dependent receptor-receptor interactions have also been reported for the adenosine A1 receptor-GFP fusion protein (A1-GFP) in purified cell membranes (Hern et al., 2004). Using fluorescence correlation spectroscopy (FCS), the aim of the current study was to investigate whether changes in density of A1-GFP expression caused changes in the molecular organisation of the receptor within small locales at the surface of living cells. 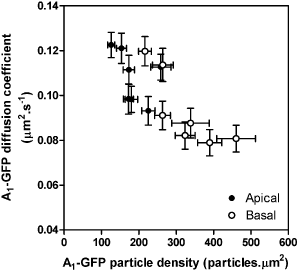
Figure 1; A1-GFP diffusion coefficient and particle density at apical and basal cell surfaces for all seven cell lines. Each data point shown is mean ± s.e.m of 33-58 cells for each cell line. FCS measurements at basal cell surfaces showed a significant. Pearson correlation (P < 0.05, r2 = 0.65) between A1-GFP density and mobility. These results show that not only is A1-GFP differentially organised at apical and basal cell surfaces (Hern et al., 2008, this meeting), the mobility of A1-GFP particles at the basal cell surface is decreased with increasing density of expression. We show that over a pharmacologically relevant range of receptor expression (Cordeaux et al., 2000) FCS identifies significant changes in the mobility and density of cell surface receptors within small regions of the surface of living cells. |
|

