062P Brighton
Winter Meeting December 2008 |
Differential association of neuropeptide Y Y1 and Y2 receptors with β-arrestin2 revealed by bimolecular fluorescence complementation (BiFC)
Laura Kilpatrick, Stephen Briddon, Stephen Hill, Nick Holliday
University of Nottingham, Nottingham, UK
Association between the neuropeptide Y (NPY) Y1 receptor and βarrestin2, respectively tagged with venus YFP N and C fragments (YN, YC), drives irreversible complementation to regenerate a fluorescence signal (Holliday et al., this meeting). Here we investigate whether the BiFC assay distinguishes Y1 receptors from the NPY Y2 receptor with reduced βarrestin2 affinity (Berglund et al., 2003), and arrestin binding properties of mutant Y receptors (Holliday et al., 2005; Marion et al., 2006).
A HEK293T clone expressing βarrestin2-YN(fragment 1-173) was additionally transfected with FLAG tagged Y receptor-YC(155-238) cDNA, to establish mixed stable YR / βarr2 populations. Cell surface receptors were confirmed in each case by FLAG antibody labelling. Confluent cells on Costar 3904 plates were incubated for 30 min at 37°C in DMEM / 0.1% BSA, and agonist then added for 60 min. Cells were fixed with 3 % PFA and the nuclei stained (H33342), before automated image acquisition (IX Ultra platereader, Molecular Devices). Granularity analysis of YR / βarr2 BiFC quantified the average intensity of 3 – 18 μm diameter vesicles, normalised to plate controls (vehicle / 1 μM NPY in native Y1R or Y2R cells). Pooled concentration-response data was analysed by Graphpad Prism v5.
Figure 1A shows that NPY stimulated Y1R / βarr2 BiFC (pEC50: 8.49 ± 0.11, n = 4) was prevented by Tyr346 truncation (ΔY346), which removes the C tail phosphorylation sites. In Y2R / βarr2 cells, NPY BiFC responses were 20-fold less potent than for the Y1R (Figure 1B; pEC50: 7.18 ± 0.06, n = 6). H155P mutation, in the 2nd intracellular loop of the Y2 receptor, increased NPY potency and maximum BiFC response (Figure 1B; NPY pEC50: 8.28 ± 0.18, n = 6), consistent with its enhancement of Y2 receptor association with βarrestin2 (Marion et al., 2006). The Y2 receptor ligand peptide YY (3-36) was also a full agonist in Y2H155P / βarr2 cells (pEC50: 8.56 ± 0.19; 1 μM response 89.0 ± 5.7 % of 1 μM NPY, n = 5), while a Y1 receptor agonist [Leu31, Pro34]NPY was inactive (3 μM ; 7.1 ± 8.6 % of 1 μM NPY, n = 5). Our novel BiFC analysis therefore accurately identifies Y receptor subtypes and mutants with different affinities for βarrestin2.
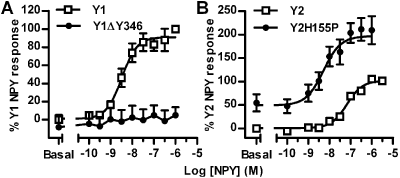
Figure 1. Y receptor / βarrestin2 interaction measured as increases in BiFC YFP intensity: (A) Y1 vs Y1βY346 and (B) Y2 vs Y2H155P (n = 4 – 6 triplicate experiments)
Financial support is acknowledged from the MRC.
Berglund MM et al. (2003) JPET. 306, 147
Holliday ND et al. (2005) Mol. Pharmacol. 67, 655
Marion S et al. (2006) J.Biol.Chem. 281, 2932
|


