104P Brighton
Winter Meeting December 2008 |
Substituted cysteine accessibility mutagenesis shows that the 5-HT3AB receptor requires an A subunit as the primary (+) ligand binding subunit
Kerry Price, Andrew Thompson, Sarah Lummis
University of Cambridge, Department of Biochemistry, Cambridge, UK
The 5-HT3 receptor is a member of the Cys-loop receptor family. Ligands bind at the interface between two subunits, with residues from both the primary (+) and complementary (-) subunit interfaces thought to be involved. In the 5-HT3AB receptor, it is unclear whether 5-HT binds to A-A, A-B, B-A or B-B interfaces to cause channel opening. Here we used substituted cysteine accessibility mutagenesis to investigate the accessibility of a residue at the + interface in 5-HT3A and 5-HT3B subunits. This residue is close to A subunit residue W178 which forms a cation-π interaction with 5-HT (Beene et al. 2002).
Voltage clamp recordings were performed on Xenopus oocytes injected with ∼5ng mRNA produced byin vitro transcription of cysteine mutant human 5-HT3A and 3B receptor subunits in pGEMHE. Oocytes were continually perfused with calcium-free ND96 and concentration-response data were collected with 5-HT (Sigma). The cysteine-reactive compound (2-aminoethyl)methanethiosulfonate (MTSEA) was applied at a concentration of 2 mM for 2 min, after which concentration-response data were again collected. For protection experiments, MTSEA was co-applied with maximal concentrations of either 5-HT (1mM) or the competitive antagonist d-tubocurarine (dTC; 1mM).
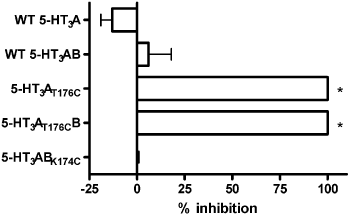
Figure 1. The effect of MTSEA on the response to a maximal concentration of 5-HT at wild type (WT) and mutant 5-HT3 receptors. Percent inhibition was calculated as (1-(I5-HTmax after MTSEA / I5-HTmax before MTSEA) × 100). Data are mean ± SEM, n = 4-6. * significantly different from WT (P < 0.01, Student’s t-test).
Treatment with MTSEA abolished the response to a maximal concentration of 5-HT at 5-HT3AT176C mutant receptors (Figure 1). This modification by MTSEA was wholly prevented by its co-application with 5-HT or d-TC. Interestingly, the same effect was seen when this mutant subunit was co-expressed with the WT 5-HT3B subunit. In contrast, MTSEA had no effect on the response to a maximal concentration of 5-HT at receptors containing the analogous mutation in the 5-HT3B subunit (K174C).
MTSEA modification of h5-HT3AT176C receptors indicates that T176C is solvent-accessible and that this disrupts receptor function. The ability to block MTSEA modification with 5-HT and d-TC demonstrates that this residue lines the binding pocket. Furthermore, the B subunit cannot restore receptor function. Taken together, these data provide strong evidence to support the hypothesis that 5-HT binds to an interface which has an A subunit as the primary (+) subunit (i.e. an A-A or an A-B interface) to cause channel opening.
Beene DL et al. (2002) Biochemistry 41 : 10262-10269
|


