106P Brighton
Winter Meeting December 2008 |
The stability of the h5-HT3A receptor complex and the importance of C-terminus
Nikolaos Batis, Amy S. Butler, Nicholas M. Barnes
Cellular and Molecular Neuropharmacology Research Group, School of Experimental and Clinical Medicine, The Medical School, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK
The 5-HT3 receptor is a ligand-gated ion channel that mediates fast synaptic neurotransmission. We have previously shown that the C-terminus of the human (h) 5-HT3A subunit is important for the expression of the homomeric 5-HT3A receptor within the cell membrane, and suggested a role for the C-terminus in the promotion of receptor stability (Butler et al., 2008). In the present study, we further assess the stability of the h5-HT3A receptor in comparison with that arising when the C-terminal alanine is truncated (ΔAla455 [N-4TM-QY]; see Butler et al., 2008 for construct terminology).
Expression of the h5-HT3A subunit and mutants/truncations by COS-7 cells and radioligand binding assays were performed as described previously (Butler et al., 2008). Briefly, COS-7 cells were transiently transfected with the appropriate cDNA via electroporation and harvested 48 h post-transfection to generate binding homogenates to assess the impact of urea upon specific [3H]granisetron binding.
[3H]Granisetron specific binding (defined by ondansetron, 10 μM) arising from expression of the truncation mutant of the h5-HT3A subunit, N-4TM-QY (ΔAla455), was more susceptible to disruption by increasing concentrations of urea relative to the specific binding arising from expression of the wild-type h5-HT3A subunit (P<0.05, Mann Whitney U test). In contrast, there was no significant difference in the urea sensitivity of [3H]granisetron specific binding arising from expression of the wild-type h5-HT3A subunit and N-4TM-AAA (Gln453Ala and Tyr454Ala).
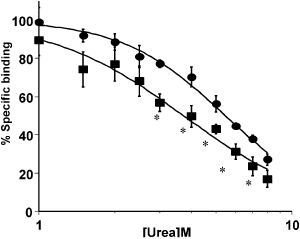
Figure 1. Specific [3H]granisetron (∼1 nM) binding levels arising from expression of the wild-type h5-HT3A subunit ( ) and the truncation mutant N-4TM-QY ( ) and the truncation mutant N-4TM-QY ( ) by COS-7 cells. Data represent mean ± SEM, n= 4. Comparison between the urea-induced inhibition of specific binding between the two constructs; *P<0.05, Mann Whitney U test. ) by COS-7 cells. Data represent mean ± SEM, n= 4. Comparison between the urea-induced inhibition of specific binding between the two constructs; *P<0.05, Mann Whitney U test.
The current study further supports the role of the C-terminus of the h5-HT3A subunit to promote stability of the arising 5-HT3 receptor complex.
Butler, AS et al., 2008. Neuropharmacology (in press).
|


