117P Brighton
Winter Meeting December 2008 |
Freely moving rat experimental model for catechol-O-methytransferase (COMT) inhibition, using microdialysis sampling
Leonel Torrao, Rita Machado, Lyndon Wright, Patricio Soares-da-Silva
BIAL- Portela & Companhia - S.A., S. Mamede do Coronado, Portugal
COMT is one of the main enzymes responsible for the metabolism of levodopa, dopamine, other catecholamines and their metabolites. Here we report the development of a freely moving rat experimental model for evaluation of COMT inhibition, using in vivo microdialysis sampling.
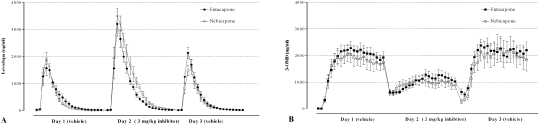
The evaluation of the COMT inhibition was based on blood circulating levodopa and 3-OMD (3 O methyldopa) concentration-time profile pharmacokinetic analysis, following daily oral administration of 12 mg/kg levodopa and 3 mg/kg benserazide concomitantly with control (day 1 and 3) or COMT inhibitor (day 2). The experimental model efficiency was evaluated using the COMT inhibitors entacapone and nebicapone. Microdialysis probes were inserted into the jugular vein of male Wistar rats (n=5, 265 ± 13 g), which were monitored for levodopa and 3 OMD over 3 experimental days (22 samples per day). Levodopa and 3 OMD quantifications were done using an high performance liquid chromatography coupled with electrochemical detection technique (adapted from Silveira et al., 2003).
Figure 1 displays the mean ± SEM levodopa (A) and 3-OMD (B) concentrations at different time points for the three different treatment periods. Using this model, entacapone and nebicapone administrations were found to reduce the formation of 3 OMD and extend the systemic exposure to levodopa, in accordance with previously published data (Nissinen et al., 1992; Soares-da-Silva et al., 2003).
In vivo probe recoveries were determined by retrodialysis (n=5, 24 ± 6 % for levodopa and 27 ± 5 % for 3-OMD). The integrity of the probes in the experimental procedure was maintained throughout the experimental procedure.
Microdialysis empowers levodopa and 3-OMD pharmacokinetic studies enabling the evaluation of the efficacy of COMT inhibitors in pre-clinical studies, minimizing human resources and material and considerably reduces the number of experimental animals required.
Nissinen et al. Naunyn Schmiedebergs Arch Pharmacol 1992; 346, 262-66.
Silveira et al. Eur J Clin Pharmacol 2003; 603-609.
Soares-da-Silva et al. Pharmacol Tox 2003; 92: 272-78.
|


