118P Brighton
Winter Meeting December 2008 |
In vitrodissolution of mycophenolate mofetil: comparison between innovator and generic formulations
Emmanuel Schuebel, Laurent Adamy
F. Hoffman-La Roche Ltd, PTDF-A: Pharma Global Technical Operation, Basel, Switzerland
Mycophenolate mofetil (MMF) is an immunosuppressive agent indicated for the prophylaxis of acute rejection in patients receiving allogeneic renal, cardiac or hepatic transplants. MMF, a weak base, is a Biopharmaceutics Classification System (BCS) class II substance (Yu et al. 2002), exhibiting a strong pH-dependent solubility profile. It is absorbed rapidly and hydrolyzed by esterases to the active metabolite mycophenolic acid. However, differences in solid-state properties, formulation and/or manufacturing processes can lead to disparities in bioavailability between brands of the same drug. As generic drugs are approved based on comparison with the innovator brand only, it is possible that switching between generic products may lead to potentially greater disparity than switching between a single generic product and the innovator brand—with potentially serious clinical consequences. As the in vivo drug dissolution is a rate-limiting factor in drug absorption for BCS class II drugs (Emami, 2006), the use of appropriately designed in vitro dissolution tests can potentially discriminate between formulations with different bioavailability. We undertook this study to compare the in vitro dissolution of the original MMF innovator brand (CellCept®, Roche) with available generic products.
Two representative batches of CellCept® 500mg tablets and 14 different generic formulations were tested using different dissolution testing scenarios simulating conditions in the proximal gastrointestinal tract. These scenarios included stomach and/or small intestine media composition, surface tension, pH, increased buffer capacity and osmolarity after food intake.
Eight of the generic formulations tested passed the quality control dissolution test (pH 1.1) according to specification Q=75% after 5 min (i.e. all single units >80% dissolved), and 12 passed the specification Q=85% after 15 min (i.e. all single units >90% dissolved). This suggests an almost homogenous dissolution rate in an acidic environment between brands. However, at pH 4.5, large variations in in vitro dissolution performance between generic formulations were observed (extremes resulting in more than 60% dissolved difference after 30 min, see figure 1). Significant variability was seen among the different generic formulations and between the various generic formulations and the original MMF innovator brand, CellCept®. Moreover, the natural variation in gastric pH in the fasted state (pH 1.0 to 4.5) may result in a strong decrease in the release rate for some generics, which may significantly delay Tmax and reduce Cmax at higher pHs because the drug may potentially not have been completely dissolved before gastric emptying occurs (15–30 minutes under fasting conditions).
In conclusion, important differences exist between the different generic formulations with regard to in vitro performance. As MMF is prescribed for life-long use, and that changes in drug performance may have serious clinical consequences (e.g. organ rejection), clinical testing is necessary to evaluate the pharmacokinetics and the impact on clinical safety of a switch between brands.
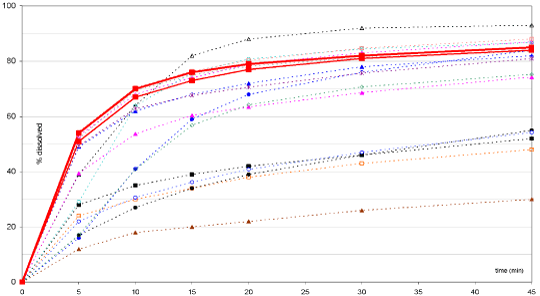
Figure 1. Dissolution profiles of CellCept (bold square) and 14 generic MMF formulations at pH4.5.
|


