Print version
Search Pub Med
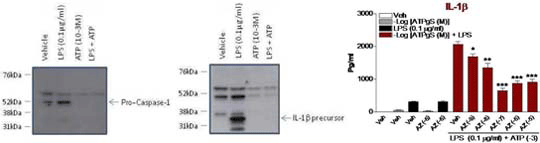
ATP acts via P2X7 receptors to active the inflammasome and enhances LPS-induced release of inflammatory cytokines. Chronic Obstructive Pulmonary Disease (COPD) is a cigarette smoke (CS) driven inflammatory disease of the airways which is increasing in prevalence worldwide whilst no effective therapies exist. Inflammasome-linked cytokines (e.g. IL-1β) are increased in animal models of COPD and clinical samples from COPD patients and thus could play a key role in the pathogenesis of the disease. We hypothesise that CS leads to the release of mediators (e.g. ATP) which activate P2X7 purinergic receptors leading to the activation of the inflammasome, cleavage of pro-caspase-1 and subsequent release of IL-1β. Before we could test the hypothesis in our CS driven in vivo model of COPD we wanted to validate tools/biochemical methods in a cell based assay.

The results demonstrated that addition of ATP results in the cleavage of pro-caspase-1 (left panel) and release of IL-1b from the cell cytoplasm (middle panel). Combining ATPγ and LPS leads to enhanced increase of IL-1β (right panel) but not non-inflammasome linked cytokines such as TNFα (data not shown). Blockade of the P2X7 receptor significantly attenuated the ATP-enhanced increase in IL-1β (One-Way ANOVA, Bonferroni correction, * (p<0.05). We have parallel data in primary macrophages from human lung tissue and in a murine cell line (with an antagonist which blocks mouse P2X7 receptor). |
|

