In vitro assessment of the interactions between acyl glucuronide drug metabolites and human serum albumin Introduction: Tolmetin (T) and diclofenac (D), members of the non-steroidal anti-inflammatory class of drugs (NSAIDs), are largely metabolized to acyl glucuronide (AG) metabolites. These AG metabolites have been postulated to be chemically unstable and protein reactive. We have investigated the chemical interactions between synthetic AG metabolites of T and D with protein, using human serum albumin (HSA). Methods: T-AG and D-AG were incubated with human serum albumin at increasing concentrations for 16 hrs at 37°C. AG covalent adduct formation was assessed using LC-MS/MS technology (AB Sciex QTRAP 5500.) Quantification of covalent HSA modification was measured using an alkaline hydrolysis method. Rates of degradation of a 2mM solution of the 1-β isomer of D-AG and T-AG was measured in both 100mM phosphate buffer pH7.4 and a 40µM solution of HSA made up in phosphate buffer, using HPLC to measure generation and loss of positional isomers. Non-covalent interactions were investigated through the use of Autodock 4 3-D modeling software (http://autodock.scripps.edu/) Results: The non-covalent docking study identified 2 major AG binding pockets within HSA containing K199 and K525. These binding pockets have previously been identified as Sudlow sites I and II. Focus docking has suggested K199 to be an important close contact to the reacting site of D-AG, and K525 for T-AG, for the 1-β isomers. Covalent modification of HSA was assessed using LC-MS/MS technology as is shown by table 1. Modification was seen to occur specifically at lysine residues, with the majority of modifications located in these Sudlow sites. Diclofenac AG has been shown to rapidly degrade in phosphate buffer at physiological conditions, with addition of HSA, at lower concentrations resulting in hydrolysis back to the aglycone. This may be due to the reported esterase potential of HSA.
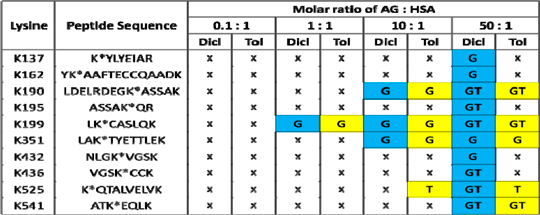
Table 1: T-AG and D-AG adduct formation with HSA following in vitro incubation at 37°C for 16 hrs. G represents adducts formed through the glycation pathway, T represents adducts formed through the transacylation pathway. Adducts were preferentially found at the hydrophobic region of HSA (Sudlow sites I and II).
Discussion: Our results have shown that both T and D-AGs can form covalent adducts when incubated with HSA in vitro via covalent modification of lysine residues. D-AG was found to degrade in buffer at physiological conditions and the addition of HSA at lower concentrations of D-AG resulted in greater levels of hydrolysis. This may be due to the reported esterase potential of HSA. In this study we have modelled both the non-covalent and covalent interactions of acyl glucuronide drug metabolites with human serum albumin.
|
|

