Print version
Search Pub Med
Characterisation of pattern recognition receptor function in human embryonic stem cell-derived endothelial cells (hESC-ECs) Human embryonic stem cell-derived endothelial cells (hESC-ECs) are currently being investigated as potential therapies in regenerative medicine for cardiovascular disease. hESCs can be manipulated to form endothelial-like cells in vitro although these cells do not respond to LPS (Foldes et al. 2010; PLoS One 2010 May 5;5(5):e10501). Thus, the full extent of how these ‘hESC-ECs’ function is not known. Endothelial cells are important immune surveillance cells and as such have highly developed pattern recognition receptor (PRR) pathways including Toll-like receptors (TLRs) and nucleotide oligomerisation domain (NOD) receptors. These are responsive to distinct pathogen associated molecular patterns (PAMPs). In the case of TLR4 and NOD1, these receptors sense LPS and peptidoglycan fragments (mimicked by synthetic ligands including C12-iE-DAP) on Gram negative bacteria, respectively. TLR4 signals through the MyD88 and TRIF whilst NOD1 signals through RIP2 each of which culminates in NF-κB activation. Here we have compared TLR4 and NOD1 signalling in hESC-ECs with those in human umbilical vein endothelial cells (HUVEC) using an high through-put Cellomics® Arrayscan® VTi HTS reader to measure NF-κB activation and measuring CXCL8 release by ELISA. Cellular responses to IL-1β were used to index the functional activity of NF-κB pathways. hESC-ECs or HUVECs responded to IL-1β or C12-iE-DAP with NF-κB translocation and release of CXCL8 (Figure 1). HUVECs, but not hESC-ECs responded to LPS with NF-κB translocation and release of CXCL8 (Figure 1). Thus, hESC-EC lack TLR4 function but retain NOD1 and MyD88 signalling pathways. Given the role of PRRs in cardiovascular disease these findings may have important implications for the optimal therapeutic use of hESC-ECs.
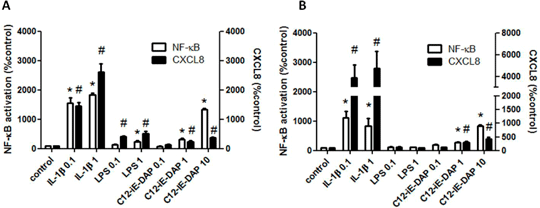
Figure 1. NF-κB (1h) activation and CXCL8 (24h) release from (A) HUVECs and (B) hESC-ECs following treatment with IL-1β (0.1-1ng/ml), LPS (0.1-1ug/ml) or C12-iE-DAP (0.1-10ug/ml). Data is normalised to respective controls and shown as mean ± SEM for n = 6-14 and analysed using one-sample t-test; *, #p<0.05.
|
|

