Comparison of non-clinical drug safety signals with nefazodone and buspirone Drug induced liver injury (DILI) is now the most common cause of acute liver failure in the developed world and is cited as the reason for 50% of all drug withdrawals from the market. Nefazodone, an anti-depressant was withdrawn from the market in 2004 following several cases of severe liver failure. The points in the cell that are vulnerable to nefazodone injury are indicated in figure 1. Our primary route of investigation has been the formation of reactive metabolite species using rat liver microsomes (RLMs) and freshly isolated rat hepatocytes (RHs). We have used buspirone as a safe, yet metabolically similar control.
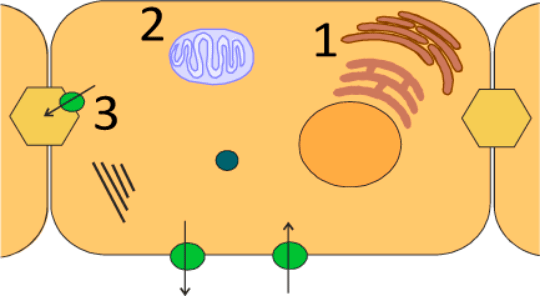
Figure 1. The Achilles heels of the cell in relation to nefazodone injury. 1) Reactive metabolite formation in the endoplasmic reticulum. 2) Inhibition of complexes of the oxidative phosphorylation chain. 3) Inhibition of the bile salt export pump.
Investigation of phase I covalent binding was carried out in RLMs. 0.1µCi of [14C]-labelled drug and 10µM cold drug were used and covalent binding assessed using a solvent extraction method. RHs were isolated from male Wistar rats using a two step collagenase perfusion method. RHs were pre-incubated for 30 mins +/- 1-aminobenzotriazole (ABT) before addition of buspirone or nefazodone. Toxicity was assessed using trypan blue exclusion assay and ATP levels. Glutathione levels over a time course of 0-6 hours in relation to toxicity were also investigated. For all experiments n = 3. Both drugs exhibited NADPH-dependent covalent binding to RLM protein compared to control. However, nefazodone showed binding almost 10-fold that of buspirone (nefazodone 105.54±21.58 pmol equiv/mg protein; buspirone 12.76±0.83 pmol equiv/mg protein). Binding was also significantly reduced when the RLMs were pre-incubated with the CYP450 inhibitor ABT. Following previous investigations the effect of ABT on toxicity was carried out using 50µM drug at a 6 hour time point and a 30min pre-incubation with ABT. Despite inhibiting the metabolism of nefazodone there was a significant increase in toxicity using both trypan blue exclusion (without ABT 102.5±8.2 % control; with ABT 65.0±9.3 % control) and ATP assay (without ABT 122.9 ± 23.9 % control; with ABT 71.1±22.5 % control) suggesting that the parent compound is more toxic than the metabolites. A time course of nefazodone and buspirone toxicity was also undertaken and compared to GSH levels in the cell. This indicated that GSH depletion was a consequence and not a cause of cell death. In conclusion, nefazodone displays covalent binding in RLMs and toxicity in RHs. Despite binding in RLMs indicating covalent binding as a source of toxicity ABT appears to increase toxicity. Future work will investigate whether mitochondrial toxicity and BSEP inhibition play a part in the toxicity of nefazodone.
|
|

