Determination of unidirectional field potential propagation using MEA chips with topographical guidance Over the last 3 decades, microelectrode array (MEA) system has been developed and improved in terms of signal-to-noise ratio, electrode patterns, and the number of electrodes on a single chip [1]. MEA chips can now be tailor-made according to the interest of the research project. The present study used biocompatible SU-8 photoresist to construct multiple topographically guided electrodes with cell reservoirs on a silicon wafer (Fig. 1). A uniform distance between each detection electrode (30 μm) along the guided tracks was fabricated. The width of the guided track of 30 μm constrained the cultured cells in an inter-connected and unidirectional fashion. Using this type of MEA chip, the unidirectional propagation speed of extracellular field potential (exFP) was then measured. Embryonic cardiomyocytes of Sprague-Dawley rats (E19-20 days) were cultured on the MEA chip with SU-8 guided channels. Spontaneous exFPs of the cells were measured after 2-4 days in culture. Chronotropic drugs, verapamil (VP, 1 nM – 10 μM) and isoproterenol (ISO, 1 nM – 1 mM), were added to the active culture for characterising the sensitivity and the functionality of the MEA. The propagation speed of exFP (264.12 ± 12.41 μm/ms, n = 12) reduced to 53.30 ± 16.2 μm/ms and increased to 566.52 ± 22.3 μm/ms in the presence of VP (1 μM, n = 10, P<0.001) and ISO (1 mM, n = 6, P<0.001), respectively. Surprisingly, the shifting of pacemaker regions during the incubation of drugs was observed. While the propagation direction fluctuated, the activation sequence of the exFP along the guided electrodes remained the same (Fig. 2). The present study demonstrates that the MEA chip with SU-8 guided channels provides an alternative platform for the study of the direction and speed of exFP propagation of cells. This MEA chip may be used to study the unidirectional exFP propagation of different excitable cells, e.g. cardiac cells and neurones, under different pathophysiological conditions.
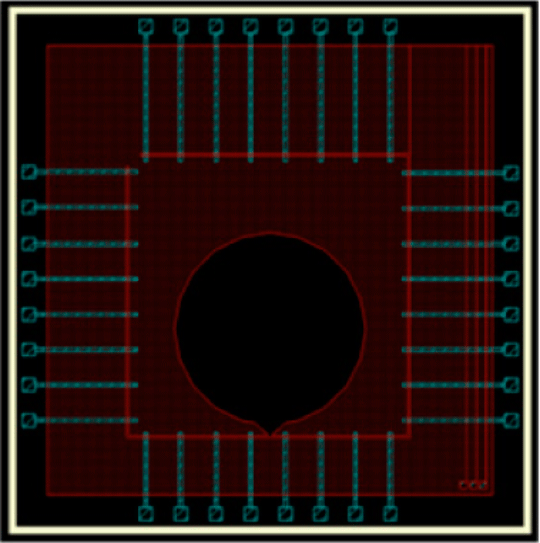
Figure 1. A schematic showing the MEA chip with multiple channels of 30 µm wide and a cell reservoir of 3000 µm in diameter. Thirty-two (8 × 4) gold detection electrodes are evently spaced (250 µm) and aligned along the guided tracks. 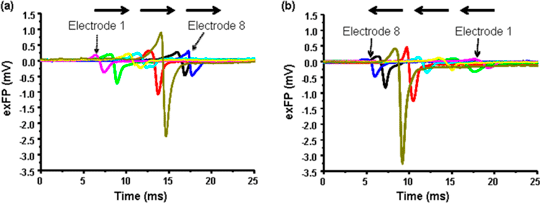
Figure 2. Representative traces of individual extracellular field potential (exFP) of cardiomyocytes detected by a row of 8 electrodes (a). The propagation direction (arrows) following the addition of verapamil (10 nM, (b)) changes, but the order of activation does not.
[1] Stett A, Egret U, Guenther E, Hofmann F, Meyer T, Nisch W, Haemmerle H. (2003): Biological application of microelectrode arrays in drug discovery and basic research. Anal Bioanal Chem., 377, 486-495.
|
|

