Immune-modulatory effects of compounds isolated from zingiber officinale roscoe (var. rubrum theilade) on immune mechanisms associated with psoriasis Psoriasis is an autoimmune inflammatory skin disease associated with aberrant activation of T and B lymphocytes. There is increasing evidence indicating that T helper 1 (Th1) and Th17 lymphocyte subsets play key roles in its immunopathogenesis: they produce pro-inflammatory cytokines and chemokines and promote the production of PGE2 and nitric oxide (NO). Further, activated Th1/Th17 cells interact with keratinocytes leading to their proliferation and hyperplasia and also promote angiogenesis; another characteristic of psoriasis. Our studies focus on developing new approaches for targeted therapy for psoriasis. In this respect, we have examined the potential therapeutic effects of compounds extracted from the ginger species, Zingiber officinale Roscoe (var. rubrum Theilade), on the mechanisms involved in psoriasis. Preliminary studies showed that the chloroform extract (HB02) of this ginger potently inhibited NO production compared to the ethanol extract (HB01, HB03; Figure 1). Hence, the effects of selected fractions and compounds from HB02 were assessed for their ability to suppress production of pro-inflammatory mediators in macrophages. Fractions, F5, F6, F7 and F10 potently suppressed NO and PGE2 production with higher potency than L-NAME. F6 was particularly potent at inhibiting NO (IC50 = 6.7± 2.7 μg/ml), and was 2-fold more effective than HB02 (IC50 = 16 ± 5.7 μg/ml) as well as suppressing iNOS gene transcription by 82.3±3.7% at 20 μg/ml. The fractions and compounds had comparable effects to inhibition of PGE2 production by indomethacin (Figure 2). Three major compounds were isolated from F6, which were F6 (3)-BI, F6 (3)-B2 and F6 (3)-B3. Among these, F6 (3)-B1 and F6 (3)-B2 appear to have additive effects in down-regulating iNOS and il23 gene transcription (Figure 3). The sum of the inhibitory effects of the two compounds appears to be equivalent to the effect of unfractionated F6, suggesting they are primarily responsible for the anti-inflammatory effects of HB02.
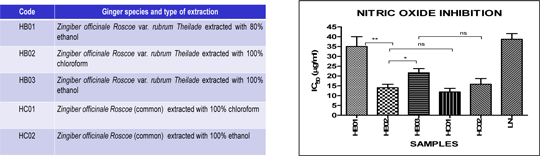
Figure 1: The IC50 of NO production by the ginger extracts and L-NAME, an inhibitor of NO. The chloroform extracts (HB02, HC01) showed the most potency.
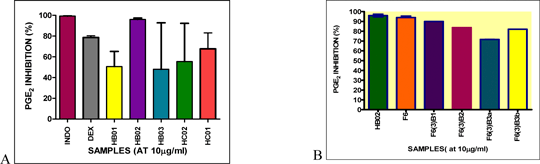
Figure 2: A) HB02, its fractions and isolated compounds had comparable effects to indomethacin (INDO) at inhibiting PGE2 production. B) Although the fractions and compounds from HB02 had less effect on PGE2 relative to HB02, their activities were comparable to INDO. Compounds F6(3) B1, -B2 and -B3 (B3a and B3b) were isolated from fraction 6 (F6) using solid phase extraction and preparative thin layer chromatography, respectively.
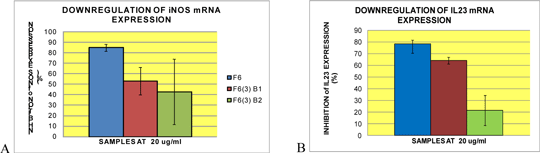
Figure 3: Compounds isolated from HB02 fraction 6 (F6) have additive effects on reducing iNOS and IL23 mRNA levels. Real time PCR show that the inhibitory activity of F6 on A) iNOS and B) IL23 mRNA levels is equivalent to the combined effects of both compounds.
|
|

