Print version
Search Pub Med
The Role of Dopamine D2, But Not D3 Or D4, Receptor Subtypes In Quinpirole-Induced Inhibition Of The Cardioaccelerator Sympathetic Outflow In Pithed Rats Lefevre-Borg et al. (1987) have previously shown that dopamine D2-like receptors inhibit the cardioaccelerator sympathetic outflow in pithed rats since: (i) quinpirole (a D2-like receptor agonist) inhibited the tachycardic responses to electrical stimulation of either the preganglionic sympathetic outflow or postganglionic cardioaccelerator nerve fibres, but not those to isoprenaline; and (ii) the antagonist sulpiride (D2-like), but not SCH 23390 (D1-like) or idazoxan (α2-adrenoceptor), blocked this effect of quinpirole. The objective of the present study was to identify pharmacologically the D2-like receptor subtypes (i.e. D2, D3 and D4) involved in the above sympatho-inhibition induced by quinpirole. After ether anaesthesia, a total of 114 male Wistar rats (240-280 g; divided into 19 groups, n = 6 each) were pithed, artificially ventilated with room air (56 strokes/min; stroke volume: 20 ml/kg) and prepared for preganglionic spinal stimulation (C7-T1; 0.03-3 Hz; 50 V; 2 ms pulses) of the cardiac sympathetic outflow or sequential intravenous (i.v.) bolus of noradrenaline (0.03-3 μg/kg), as reported by Sánchez-López et al. (2003). The carotid artery was cannulated for measurement of blood pressure and heart rate. Prior to electrical stimulation, all animals received i.v. gallamine (25 mg/kg; to avoid electrically-induced muscular twitching) and desipramine (50 μg/kg; to amplify the sympatho-inhibitory responses at lower frequencies of stimulation). The body temperature was kept at 37°C by a lamp and monitored with a rectal thermometer. Data analysis included 2-way repeated-measures ANOVA+Student-Newman-Keuls test. Statistical significance (*) was accepted at P<0.05. Electrical stimulation of the cardiac sympathetic outflow (control responses; Figure 1) and i.v. noradrenaline (not shown) resulted in frequency-dependent and dose-dependent tachycardic responses, respectively. These responses remained unchanged (P>0.05) after i.v. infusions of saline (0.02 ml/min). In contrast, i.v. infusions of quinpirole (0.1-10 μg/kg.min) dose-dependently inhibited the sympathetically-induced tachycardic responses (not shown). 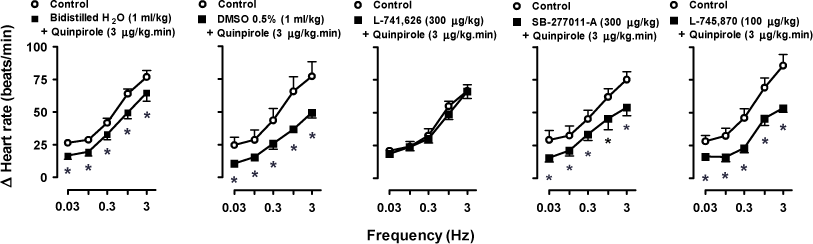
Figure 1. Effect of vehicles or antagonists on quinpirole-induced cardiac sympatho-inhibition. Moreover, Figure 1 shows that the cardiac sympatho-inhibition induced by 3 μg/kg.min of quinpirole (which did not affect the responses to noradrenaline; not shown): (i) remained unchanged after i.v. injections of the vehicles [1 ml/kg of bidistilled H2O or 0.5% dimethylsulphoxide (DMSO)] or the antagonists SB-277011-A (D3; 300 μg/kg) and L-745,870 (D4; 100 μg/kg); and (ii) was abolished by the D2 receptor antagonist L-741,626 (300 μg/kg, i.v.). The doses of these antagonists, which had no effect per se (P>0.05) on the sympathetically-induced tachycardic responses (not shown), were high enough to completely block their respective receptors. In conclusion, the cardiac sympatho-inhibition by 3 μg/kg.min of quinpirole in pithed rats involves the dopamine D2 receptor subtype, with no evidence for the involvement of the D3 or D4 subtypes.
Lefevre-Borg, F. et al. (1987). Fundam. Clin. Pharmacol., 1, 179-200. Sánchez-López, A. et al. (2003). Br. J. Pharmacol., 140, 725-735.
|
|

