Print version
Search Pub Med
Anti-tussive Effects Of Fatty Acid Amide Hydrolase Inhibition: Evidence For Elevated Fatty Acid Amides Acting Via Cannabinoid Receptors On Airway Sensory Nerves Cough is arguably the most common symptom associated with pulmonary diseases, such as asthma, bronchitis, chronic obstructive pulmonary disease, the common cold and chronic cough. Treatment therapies for cough are limited and recent evidence identified that over the counter cough medications are ineffective. Fatty acid amide hydrolase (FAAH) is an integral membrane enzyme that hydrolyses the endocannabinoid anandamide (arachidonylethanolamide – AEA) and related amidated signaling lipids (N-oleoylethanolamine – OEA, linoleoyl ethanolamide – LEA, and palmitoylethanolamide – PEA). Pharmacological inactivation of FAAH produces analgesic and anti-inflammatory phenotypes, without showing the undesirable side effects of direct cannabinoid receptor agonists. Since cannabinoid receptor agonists have been shown to possess anti-tussive activity in pre-clinical models of cough, we hypothesised that inhibition of FAAH may represent a target for the development of novel anti-tussive agents. In vivo & ex vivo experiments were conducted in male Dunkin-Hartley guinea pigs (300-500g, Harlan and B&K Universal, UK). Administration of the FAAH inhibitor (FAAHi), PF-04862853 (Meyers et al. 2011) (1.0 and 10.0 mg kg-1 p.o.) resulted in significantly elevated plasma and brain levels of the fatty acid amides (FAAs) – AEA, OEA, LEA and PEA – which reached a maximum at 4h. With this dosing regimen, pharmacological inactivation of FAAH with PF-04862853 significantly inhibited citric-acid-evoked cough using conscious guinea pigs, (48%±16, P<0.05 and 53%±13, P<0.01, respectively, n = 8 per group). PF-04862853 (0.3 – 10 µM) caused a concentration-related inhibition of both capsaicin- (1 µM) and low pH (pH 5)-induced depolarisation of guinea pig isolated vagus nerve. In addition, the closely related analogue PF-04457845 (Johnson et al. 2011) (0.01 – 10 µM) reduced capsaicin- and low pH-induced depolarisations of rat and guinea pig isolated vagus nerves in a concentration-related manner. Furthermore, PF-04457845 (0.1 µM) in human isolated vagus nerve inhibited capsaicin- and low pH-induced depolarisations (55 and 67% respectively, n = 1). These inhibitory effects of both FAAHi’s were significantly reversed (Kruskal-Wallis with Dunn’s post-test, n = 4, *p<0.05) by the cannabinoid CB2-receptor antagonist, SR144528 (0.01 µM), but not by the cannabinoid CB1-receptor antagonist, SR141716A (0.01 µM) (figure 1). Since inhibition of FAAH elevates several FAAs, we examined the effects of AEA, OEA, LEA and PEA on capsaicin- and low pH-induced depolarisations of guinea pig isolated vagus nerve. At a concentration of 0.1 µM, all 4 FAAs caused substantial inhibition (60 – 80%) of the induced depolarisations, an effect that was reversed by the CB2-receptor antagonist, SR144528, in all cases (figure 2). To further examine the mechanism of inhibition of vagus nerve depolarisation by the FAAs, we selected PEA (0.1 µM) to investigate the effects of various potassium channel blockers. Inhibition of both capsaicin- and low pH-depolarisations of guinea pig isolated vagus nerve by PEA were almost completely reversed by apamin – an inhibitor of small conductance Ca2+-activated K+ (SK) channels (see figure 2). Preliminary data suggests that this latter observation also translates to human isolated vagus nerve, where PEA inhibited low pH-induced depolarisation by 62%, which was reversed by apamin (n = 1). 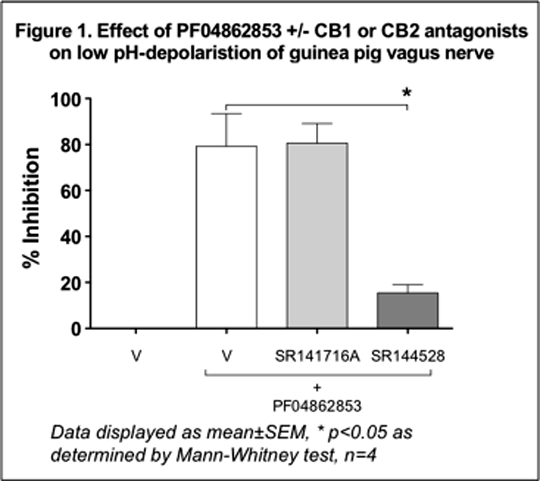 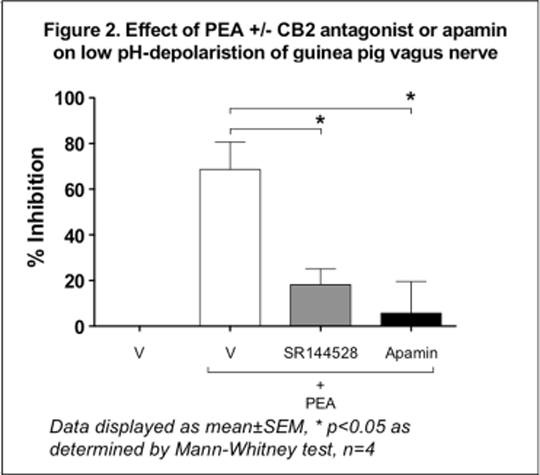
This study demonstrates, for the first time, that inhibition of FAAH produces anti-tussive activity in conscious guinea pigs. Furthermore, our data indicate that this effect could be mediated by elevated FAAs, acting on cannabinoid (CB2) receptors present on vagal sensory nerves, leading to activation of SK channels. These findings identify FAAHi as a potential target for the development of novel, safe therapeutic agents for the treatment of cough.
Johnson, D.S. et al., 2011. ACS medicinal chemistry letters, 2(2), pp.91–96. Meyers, M. et al., 2011. Bioorganic & medicinal chemistry letters. (In press).
|
|

