Print version
Search Pub Med
Adrenomedullin; A Structural Paradigm Shift in Class B G-Protein Coupled Receptor Peptide Hormones? The Calcitonin Gene-Related Peptide family of Class B G-Protein Coupled Receptors (GPCRs) are important in many cellular and developmental processes. The peptide hormone adrenomedullin (AM) is the ligand for the Calcitonin Receptor-Like Receptor (CLR) and its complex with Receptor Activity-Modifying Proteins (RAMP) 2 and 3, generating AM1 and AM2 receptors respectively. AM activation of these receptors has many downstream effects including the development of the lymphatic and blood vascular systems and the protection of the cardiovascular system in cardiovascular disease. The class B GPCR family are characterised by a large N-terminal extracellular domain (ECD) which binds the C-terminal portion of peptide hormones. The AM peptide fragment AM22-52 is an antagonist of AM receptors and its administration has been shown to reduce endothelial cell proliferation, migration, angiogenesis and tumour vascularisation most likely via the AM1 receptor. In this study we sought to determine the structure of AM22-52 in its solution and receptor-bound forms in order to map its receptor binding characteristics. The ECD of the AM1 receptor was expressed recombinantly as insoluble material in Escherischia coli and was refolded and purified using ion-exchange and size exclusion chromatography. Correct folding and binding of AM22-52 was confirmed by isothermal titration calorimetry (ITC), Kd = 5μM ± 1, n = 2. AM22-52 for NMR analysis was synthesised using Fmoc protected 13C and 15N labelled amino acids and used in heteronuclear NMR analysis. Comparison of the 2D 1H-15N HSQC spectra of AM22-52 in its solution and ECD bound forms showed chemical peak shifts corresponding to the C-terminal nine residues of the peptide (Ser45-Tyr52), suggesting binding of these residues to the receptor. Some shifts were also seen for the Gln24-Thr34 region. To confirm the importance of the C-terminus, the binding of selected alanine mutants of this region of AM22-52 to the ECD was analysed by ITC. Differential effects were observed. Wild type AM22-52 binding was completely abolished by G51A, whereas Q50A only decreased binding affinity 2-fold (Kd = 10μM±1, n = 2). The structure of AM22-52 in its receptor ECD-bound and solution states was then determined by heteronuclear trNOESY NMR spectroscopy, revealing structures with no stable secondary structure. In solution, one form predominated (overall RMSD = 0.9Å). In keeping with current class B GPCR peptide hormone-receptor binding dogma, changes occurred on binding to the ECD (Figure 1), with the N-terminal potentially showing mobility and the extreme C-terminal residues presented for binding to the receptor. 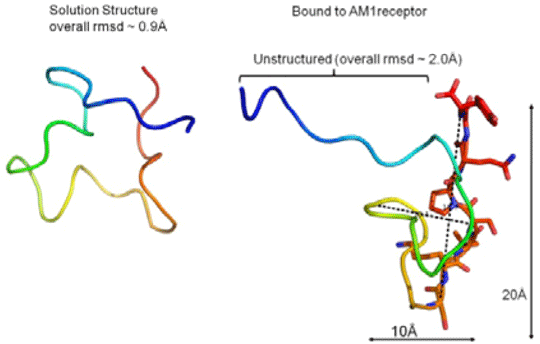
Figure 1. The NMR structures are coloured in rainbow from blue, N-terminal to red, C-terminal. A. Structure of AM22-52 in solution. B. Structure of AM22-52 bound to the AM1 receptor ECD. The nine C-terminal residues involved in binding to the ECD are shown as sticks. NMR analysis of the receptor bound form of AM22-52 shows substantial involvement of the extreme C and N-termini in receptor binding, pinpointing key regions of AM22-52 involved. The potential mobility of the N-terminal may suggest that receptor interactions in this region are transient in nature or that the binding of this region is compromised by the absence of the rest of the AM1 receptor. Although still conforming to the two domain model for peptide-class B GPCR binding, receptor bound AM22-52 shows none of the high alpha helical content exhibited by the rest of the family, even though full-length AM in SDS micelles has a small helical region around residues 22-341. Although this in itself could be due to the experimental conditions employed. This apparent departure from the current dogma of class B GPCR-peptide structures raises the question of whether RAMPs influence not just receptor conformation and potency but also the structure and mode of binding of the AM peptides, potentially setting these receptors apart from the rest of the class B family. Alternatively, we are observing transitional peptide structures that may become more helical in the presence of the receptor transmembrane domain.
(1) Pérez-Castells J., et al. 2012 Biopolymers, 97, 45-53
|
|

