A Key Role For The Endothelium In NOD1 Mediated Pathogen Recognition By Blood Vessels Systemic and pulmonary vascular dysfunction is a prominent feature of severe bacterial sepsis. Sepsis is mediated by the activation of pattern recognition receptors (PRRs) by pathogen associated molecular patterns (PAMPs) including Nucleotide oligomerisation domain-1 (NOD1) and Toll like receptor (TLR)4. NOD1 recognises meso-diaminopimelic acid containing peptidoglycan from predominantly Gram negative bacteria, whilst TLR4 recognises lipopolysaccharide (LPS) from Gram negative bacteria. We have shown previously that a specific NOD1 agonist reproduces septic shock in vivo[1] and induces vascular dysfunction in vitro via induction of nitric oxide synthase (NOS) in vessels. We have shown that NOD1 mediated NOS induction in vascular smooth muscle is via NFκB, MAPK and receptor interacting protein 2 (RIP2) pathways[2]. The role of endothelium versus smooth muscle in whole vessel sensing of PAMPs is not clear. Thus, in this study we have investigated the relative importance of the endothelial layer in NOD1 versus TLR4 mediated activation of whole rat aorta. Male Sprague Dawley rats were killed by isoflurane anaesthesia and cervical dislocation. The thoracic aorta was removed and dissected clean of connective tissue and cut into rings 2-3mm in length. In some rings the endothelium was removed mechanically. Rings were incubated in Dulbecco’s Modified Eagle Medium (DMEM) with 10% foetal calf serum for 1 hour before media was replaced and the NOD1 agonist Lauroyl-γ-D-glutamyl-meso-diaminopimelic acid (C12-iE-DAP) 1µg/ml or the TLR4 agonist LPS1µg/ml added. Release of NO (via its breakdown product, nitrite using the Griess assay) by aortic tissue in organ culture was measured at 24 and 48h. Both C12-iE-DAP (NOD1) and LPS (TLR4) induced significant nitrite release in endothelium intact aorta compared to control at 24 hours (3.9±1.2 vs 9.4±1.5 (C12-iE-DAP) and 10.0±1.9 (LPS). P<0.05, one-way ANOVA followed by Bonferroni’s Multiple Comparison Test) and 48 hours (6.0±1.6 vs 13.4±1.8 (C12-iE-DAP) and 13.9 ± 0.8 (LPS) P<0.05, one-way ANOVA followed by Bonferroni’s Multiple Comparison Test). Removal of the endothelium significantly reduced nitrite release in response to C12-iE-DAP and LPS at 24 hours (5.5±1.7 vs 9.4±1.5 (C12-iE-DAP) and 5.5±1.4 vs 10.0±1.9 (LPS). P<0.05, two-way ANOVA followed by Bonferroni’s post tests. At 48 hours nitrite release in response to C12-iE-DAP or LPS was not significantly different between endothelium intact and denuded aortic rings (10.6±1.4 vs 13.4±1.8 (C12-iE-DAP) and 11.2±1.8 vs 13.9 ± 0.8 (LPS) P>0.05, two-way ANOVA followed by Bonferroni’s post tests) (Figure 1). Figure 1 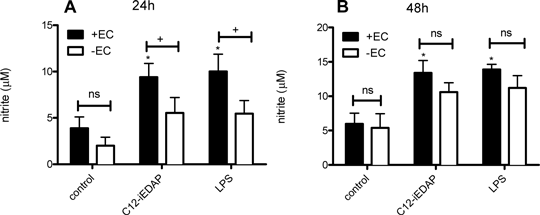
Figure 1: Induction of nitrite accumulation in conditioned media of rat aorta with (+EC) or without (-EC) endothelium at 24 (A) or 48 (B) hours. The data is the mean ± S.E.M. for tissue from n = 9 animals. Data was analysed by one-way ANOVA followed by Bonferroni’s Multiple Comparison Test; *p<0.05 or by two-way ANOVA followed by Bonferroni’s post tests; +p<0.05. This data corroborates the idea that NOD1 and TLR4 pathways co-exist in blood vessels and reveals the importance of the endothelium in initial sensing of PAMPs.
1. Cartwright, N., et al., Selective NOD1 agonists cause shock and organ injury/dysfunction in vivo. Am J Respir Crit Care Med, 2007. 175(6): p. 595-603. 2. Moreno, L., et al., Nucleotide oligomerization domain 1 is a dominant pathway for NOS2 induction in vascular smooth muscle cells: comparison with Toll-like receptor 4 responses in macrophages. Br J Pharmacol, 2010. 160(8): p. 1997-2007.
|
|

