Development of a HDM-driven model of allergic asthma. The global prevalence of asthma is increasing, and many patients’ symptoms are inadequately controlled (Chapman et al., 2008). Improved therapies are urgently needed. Pre-clinical models which closely mimic clinical features of asthma are useful to improve understanding of disease mechanisms and to identify novel therapeutic targets. We sought to develop a model of allergic asthma, utilising the clinically relevant aeroallergen: house dust mite (HDM). Previously we have shown that a single topical HDM challenge induces airway inflammation without prior sensitisation – termed here as a “non-allergic” model (De Alba et al., 2010). Presently, the aim was to develop a protocol and titrate a challenge of HDM that induces inflammation only when associated with prior sensitisation - termed here as an “allergic” model. Experiments were performed in accordance with the UK Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act 1986. A sensitisation dose and route was selected using plasma IgE as a marker of allergic sensitisation. Male C57Bl/6 mice (18-20g, n = 6-12) were sensitised on days 0 and 14, with vehicle or 0.005-500μg.kg-1 HDM♠ topically, in saline (50μl per mouse, intranasally (i.n.) under isoflurane anaesthesia), or systemically: intraperitoneally (i.p.) (in saline, 100μl per mouse) or i.p. with AlumTM (Aluminium hydroxide and magnesium hydroxide, diluted 1:1 in saline, 100μl per mouse). Total and HDM specific IgE were measured in plasma on day 21 (by ELISA). Topical sensitisation did not increase total or HDM specific IgE levels. In mice sensitised to HDM systemically, (both with and without AlumTM), a bell-shaped increase in total and HDM-specific plasma IgE was observed. HDM-specific IgE responses peaked at a sensitisation dose of 0.5μg.kg-1 HDM plus AlumTM, which was picked for further model development. Absorbance values were: AlumTM - 0.349±0.173; AlumTM plus HDM (0.5μg.kg-1) - 1.93*±0.42 (mean±SEM). * = p<0.05 (Kruskal-Wallis one-way ANOVA followed by Dunns Multiple Comparison test). To establish a challenge dose, mice (n = 8) were sensitised with AlumTM or HDM plus AlumTM as previously. Mice were challenged (days 24-26 (i.n., under isoflurane anaesthesia)) with saline (50μl) or HDM (0.125-125μg.kg-1). Bronchoalveolar lavage fluid (BALF) was obtained 24 hours after final challenge. In AlumTM–sensitised mice, total cellular inflammation in BALF was increased following high dose HDM challenge (125μg.kg-1) only. Conversely, in HDM (plus AlumTM)-sensitised mice, a dose-dependent increase in BALF total cells was observed following HDM challenge; increased levels of eosinophils in BALF following HDM challenge were only observed in mice sensitised with AlumTM + HDM (Figure 1B), illustrating the allergic nature of this response. A challenge dose of 1.25μg.kg-1, where no non-allergic inflammation was observed, was selected to further profile this model. The dependence of this allergic airway inflammation on an adjuvant (AlumTM) was then evaluated; response to HDM challenge (1.25μg.kg-1) was assessed in mice (n = 6) sensitised as previously, with, saline, HDM (in saline), AlumTM, or HDM (in AlumTM). HDM challenge resulted in allergic airway eosinophila following sensitisation both in the absence and presence of AlumTM (Figure 2). The use of a non-physiological adjuvant is therefore not required to evoke an allergic response to HDM. Further investigation will determine whether this allergic airway eosinophilia is accompanied by other key clinical features of allergic asthma, such as early and late asthmatic responses, or airway hyperresponsiveness. This model of HDM-induced allergic airway inflammation could provide an opportunity to develop novel targets for allergic asthma.
♠All quoted HDM concentrations refer to Der p 1 content.
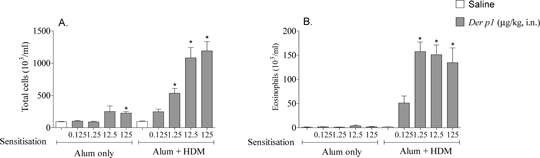
Figure1: Effect of HDM on airway inflammation in non-sensitised and sensitised mice. (A) Total white cells in BALF, (B) Eosinophil number in BALF. Male C57Bl/6 mice (n = 8) were sensitised with AlumTM, or 0.5μg ml -1 HDM in AlumTM on days 0 and 14 (in 100μl, i.p.). Mice were challenged on days 24, 25, 26 with saline or HDM (1.25μg.kg-1 in 50μl, i.n.). Inflammation in BALF was assessed 24 hours after final challenge. Data expressed as mean±SEM, p<0.05 compared to respective saline challenged controls, Kruskal-Wallis one way ANOVA followed by Dunns Multiple Comparison test.
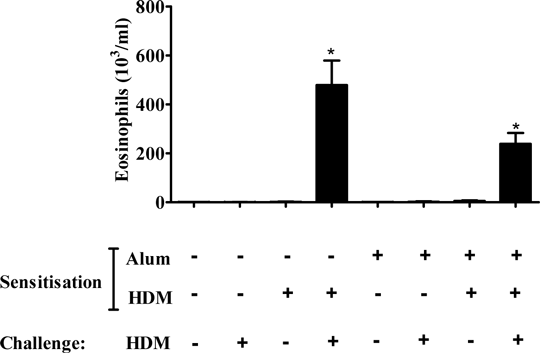
Figure 2: Effect of HDM challenge in mice sensitised to HDM in the absence or presence of AlumTM Male C57bl/6 mice (n = 6) were sensitised with saline, AlumTM, 0.5μg.kg-1 HDM in saline, or 0.5μg.kg-1 HDM in AlumTM on days 0 and 14 (in 100μl, i.p.). Mice were then challenged on days 24, 25, 26 with saline or 1.25μg.kg-1 HDM (in 50μl, i.n.). Data show eosinophil number (cells 103 ml-1) in BALF 24 hours after final challenge, expressed as mean ± SEM, p<0.05 compared to respective saline challenged controls, Kruskal-Wallis one way ANOVA followed by Dunns Multiple Comparison test.
Chapman, KR, Boulet, LP, Rea, RM, et al., European Respiratory Journal 31, 320 -325 (2008). De Alba, J, Raemdonck, K, Dekkak, A et al., Eur. Respir. J 35, 1377-1387 (2010).
|
|

