Non-competitive antagonists active at cys-loop receptors inhibit ELIC and GLIC Cys-loop receptors are eukaryotic ligand-gated ion channels (LGICs) that mediate fast synaptic transmission in vertebrate and invertebrate nervous systems. Crystallisation of homologous LGICs from prokaryotes Erwinia chrysanthemi (ELIC) and Gloeobacter violaceus (GLIC) has provided insight into the structure of Cys-loop receptors (1, 2, 3). We took non-competitive antagonists (NCAs) that act at eukaryotic receptors and examined their effects on ELIC and GLIC. ELIC and GLIC were expressed in Xenopus laevis oocytes and tested using two-electrode voltage clamp electrophysiology. Receptors were activated with concentrations of GABA (ELIC) or protons (GLIC) that gave half-maximal responses, and then co-applied in the presence of NCAs, yielding concentration-inhibition curves, from which IC50 values were derived. To identify possible NCA binding sites, ligands were docked into receptor crystal structures using in silico methods (4). 10 docking runs were performed for each compound using GOLD 3.0 and the results visualised using PyMOL v1.3 and ViewerLite v5.0. 19 LGIC (glycine, nACh, 5-HT3A and GABAA receptors) NCAs were tested. 10 compounds, including the GABAA and glycine NCA, picrotoxinin (PXN; ELIC IC50: 87 μM; GLIC IC50: 2.6 μM), inhibited both receptors. 5 compounds were selective; for example, lindane had high affinity for GLIC (IC50: 0.23 μM) but was ineffective at ELIC (< 100 μM). 4 compounds were ineffective at both receptors (< 100μM). Docking showed that the 2’ and 6’ residues of ELIC and GLIC interact with NCAs; locations previously reported at eukaryotic receptors (5, 6; Fig. 2).
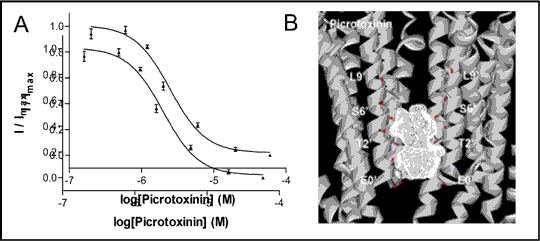
We demonstrate that ELIC and GLIC have distinct pharmacologies. In silico docking indicates that the binding sites for NCAs are similar to those of eukaryotic receptors, suggesting that the prokaryote structures can be more widely applied to eukaryotic receptors.
1. Bocquet et al. 2009 Nature, 457, 111 2. Hilf, RJ & Dutzler, R 2008 Nature, 452, 375 3. Hilf, RJ & Dutzler, R 2009 Nature,457, 115 4. Thompson et al. 2011 Mol Pharmacol, 80, 183. 5. Yang Z et al. 2007 J Neurochem., 103, 580. 6. Alqazzaz et al., 2012 Biophys J., In Press.
|
|

