Short-Term Regulation of Aquaporin 4 and its Potential Role as a Drug Target for Brain Oedema Aquaporin 4 (AQP4) is a tetrameric water channel protein with a water-conducting pore in each monomer (Fujiyoshi, 2011). AQP4 is the most abundant water channel protein in the brain, highly expressed in astrocytes. AQP4 knockout mice are protected against cytotoxic brain oedema (Verkman et al., 2006), which is where the blood brain barrier remains intact and the oedema is thought to form as a result of deterioration of cellular metabolism and retention of sodium and water. There is no generally accepted current therapy for cytotoxic oedema, in part due to a lack of understanding surrounding the underlying mechanism. Work from our laboratory on a novel trigger for sub-cellular redistribution of the homologous AQP1 kidney protein (Conner et al., 2012), (Conner et al., 2010) has allowed us to investigate this phenomenon in AQP4. We have investigated the cellular relocalisation of GFP-tagged AQP4 exposed to a hypotonic extracellular environment transfected into a live human embryonic kidney (HEK293) cell line using confocal microscopy. Relative membrane expression (RME) of the AQP-GFP was measured by comparing the fluorescence intensity profiles of lines drawn across the membrane from cells exhibited to isotonic and hypotonic extracellular environments. These lines were drawn across the cell without bisecting the nucleus and the profile intensities and surface area of the cell were calculated using the software, ImageJ, as described previously (Conner et al., 2012). AQP4 rapidly and reversibly translocated to the cell surface in response to hypotonicity, increasing RME from 29.3% +/- 6.4% to 54.9% +/- 6.6% (p<0.05; N = 3). The cellular signalling required for this translocation response was investigated by exhibiting cells to different activation inhibitors. PKAi, cytochalasin D, and extracellular calcium-free media were found to fully prevent the translocation and functional swelling response of HEK293 cells transfected with AQP4 (p<0.01; N = 3 in all cases). A model describing this phenomenon is shown in Figure 1. Structure/function analysis using site-directed mutagenesis identified a highly conserved serine residue of transmembrane region 1 (TM1) at position 52, which, when mutated to aspartic acid (S52D) lost the hypotonicity-induced translocation and cell volume increase (p<0.01; N = 3 in all cases). The alanine substitution (S52A) was unaffected. 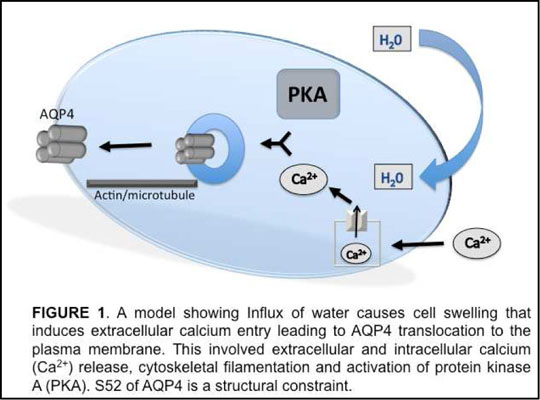
This study demonstrates that a cellular signalling response to a change in tonicity of the cellular environment leads to AQP4 translocation to and from the cell surface. This involves the influx of extracellular calcium, the activation of PKA and cytoskeletal reorganization. A key residue of AQP4 involved in this process is also described.
1. CONNER, M. T., CONNER, A. C., BLAND, C. E., TAYLOR, L. H., BROWN, J. E., PARRI, H. R. & BILL, R. M. 2012. J Biol Chem. 2. CONNER, M. T., CONNER, A. C., BROWN, J. E. & BILL, R. M. 2010. Biochemistry, 49, 821-3. 3. FUJIYOSHI, Y. 2011.. J Electron Microsc (Tokyo), 60 Suppl 1, S149-59. 4. VERKMAN, A. S., BINDER, D. K., BLOCH, O., AUGUSTE, K. & PAPADOPOULOS, M. C. 2006.. Biochim Biophys Acta, 1758, 1085-93.
|
|

