Production of CLR and RAMPs ectodomains in Pichia pastoris The calcitonin receptor-like receptor (CLR) is a family B GPCR that forms heterodimeric complexes with the receptor activity modifying proteins (RAMPs) to constitute the receptors for CGRP (CLR+RAMP1) and adrenomedulin (CLR+RAMP2, AM1 receptor; CLR+RAMP3, AM2 receptor) (McLatchie et al., 1998). Molecules targeting these receptors have been targeted for a number of conditions including migraine (CGRP receptor). Attempts to produce stable GPCRs for structural study have been faced with difficulties, usually posed by the transmembrane domain. Successes have however been recently recorded in the production of some family B GPCR and RAMP ectodomains in Escherichia coli for structural studies (Koth et al., 2010). In this study, the ectodomains of the CLR and RAMPs have been successfully produced in the methylotrophic Pichia pastoris to further investigate their structural interaction. Amplified genes encoding the extracellular N-terminal domains of the CLR and RAMPs [CLR(23-133), RAMP1(26-117), RAMP2(43-145) and RAMP3(27-119)] were subcloned into the pPIC9K_MepNet plasmid vector in frame with an α-factor secretion signal to initiate secretion of these proteins into the expression medium. The expression constructs, following transformation by electroporation, were expressed in P. pastoris cells. Secreted receptor proteins were purified with Ni-NTA columns using the batch method, as previously described (Singh et al, 2010). Purified proteins were analysed by SDS PAGE and western blot using an anti-his antibody and were further subjected to biophysical characterization, via analytical ultracentrifugation (AUC) and circular dichroism (CD) (Oates et al., 2008) . 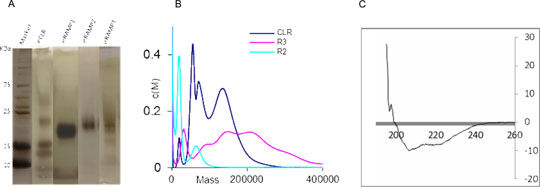
Figure 1: A) Silver-stained SDS gel of eCLR and eRAMPs. B) AUC graph showing peaks that reflect the bands observed on the silver-stained gel. C) Representative far UV CD spectra of the eRAMP2 indicating folded protein. SDS PAGE analysis revealed the purified proteins at their correct calculated molecular weight (Figure 1). This corresponds with that obtained from the western blot analysis. Although multiple bands were observed for the eCLR and eRAMP3, these may be as a result of oligomerization or protein aggregation or both. Analytical ultracentrifugation of the purified protein samples further gave peaks that correlate with those from the SDS PAGE analysis. The AUC and CD performed on these receptor proteins confirm the success of this strategy employed in their production, showing correctly folded protein of the appropriate molecular weight. The use of a eukaryotic expression system in this study offers an advantage of posttranslational modification, especially protein folding. This should give a better representation of these proteins in the anticipated mammalian system while studying their structural mechanism of interaction. Koth CM, Abdul-Manan N, Lepre CA et al. (2010). Refolding and In Vitro Characterization of a Soluble Antagonist Binding Domain of the CGRP Receptor. Biochemistry 49(9): 1862-1872. McLatchie LM, Fraser NJ, Main MJ et al. (1998). RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393(6683): 333-339. Oates J, Hicks M, Dafforn TR, et al., (2008). In vitro dimerization of the bovine papillomavirus E5 protein transmembrane domain. Biochemistry 47(34):8985-92. Singh S, Hedley D, Kara E et al. (2010). A purified C-terminally truncated human adenosine A2A receptor construct is funtionally stable and degradation resistant. Protein Expression and Purification 74: 80 - 87
|
|

