Effect of cigarette smoke exposure on a preclinical model of allergic asthma Nicole Dale1, Kristof Raemdonck1, Anthony T Nials2, Maria G Belvisi1, Mark A Birrell1. 1Imperial College London, London, UK, 2GSK, Stevenage, UK.
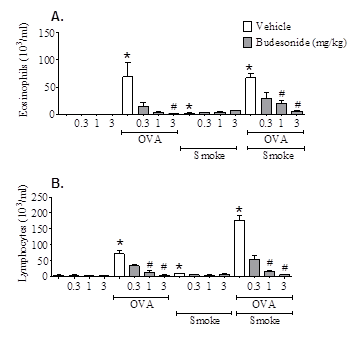
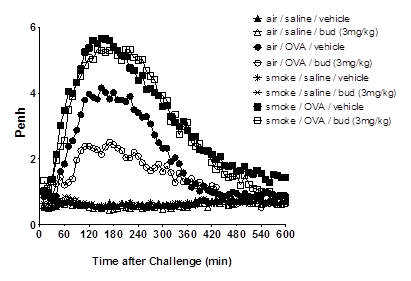
Asthmatics exposed to inhaled pollutants such as cigarette smoke (CS) have increased symptom severity (Jang et al., 2009, Siroux et al., 2000) and reduced response to corticosteroid treatment (Chalmers et al 2002, Chaudhuri et al., 2003). Approximately 25% of adult asthmatics are thought to be active smokers and many sufferers are exposed to high levels of inhaled pollutant (reviewed in Ezzatti et al., 2002). The mechanism by which CS or pollutants may alter the disease phenotype or the effectiveness of steroid treatment in asthma is not known. The aim of this study was to determine the impact of CS exposure on the phenotype and steroid sensitivity of an ovalbumin-driven model of allergic asthma. Experiments were performed in accordance with the UK Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act 1986. Male C57bl6 mice (18-20g) were sensitised with ovalbumin (OVA) plus AlumTM (day 0 and 14, 10μg in 100μl, i.p.), followed by 3 topical OVA challenges (i.n., days 24-26, 50μg in 50μl). Prior to OVA challenge, mice were exposed to CS (or air) for 3 consecutive days (b.i.d), and exposures continued until endpoint assessment. Budesonide (0.3-3 mg kg-1) was dosed orally (b.i.d) on each of the challenge days, and until endpoint assessment. Inflammation and airway hyperresponsiveness (AHR) was assessed 72 hours after final OVA challenge. CS exposure was also combined with a similar OVA-driven model, featuring the late asthmatic response (LAR). CS exposure did not impact upon the efficacy of budesonide on OVA-induced inflammation in the broncho-alveolar lavage fluid (BALF); eosinophil and lymphocyte numbers were significantly reduced by budesonide in mice exposed to OVA alone, and in combination with CS (n=7-8, p<0.05, Kruskal-Wallis one way ANOVA followed by Dunns Multiple Comparison test; Figure 1). In mice exposed to CS, OVA-induced AHR was completely abolished (n=12). Inhaled 5-HT (3 Mg.ml-1) Penh values were: Saline/air – 1223 ± 58; OVA/air – 1789 ± 129; Saline/CS – 1138 ± 51; OVA/CS – 1076 ± 62 (area under curve, mean ± SEM). The OVA-induced LAR was substantially reduced by budesonide; however, in CS co-exposed mice, budesonide failed to impact upon the LAR (n=5-8; Figure 2). CS exposure had disparate effects on the asthmatic phenotype in these models. This data has strong clinical parallels: CS exposure did not impact on the anti-inflammatory efficacy of steroids but attenuated AHR (Meghji et al., 2011). To our knowledge, the effect of smoking on steroid treatment of allergen-induced LAR has not been studied in asthma patients; however, if replicated clinically, our findings could have implications for the treatment of asthma in smoking patients. This model may be useful for investigating how CS and other airborne pollutants impact upon the asthma phenotype and its treatment, and identifying novel targets for treatment-resistant asthma.
Figure 1: Effect of budesonide on airway inflammation following OVA CS, or in combination. (A) Eosinophil number in BALF. (B) Lymphocyte number in BALF. OVA-senstised mice (Male C57Bl/6) (n=8) were challenged with saline or OVA (i.n. under isoflurane anaesthesia). Mice were also exposed to CS (or air) and treated with oral vehicle or Budesonide (0.3-3 mg kg-1). Inflammation was assessed 72 hours after final OVA challenge. Data expressed as mean±SEM * = p<0.05 vs air/saline challenged controls, Mann Whitney test. # = p<0.05 vs respective vehicle treated controls, Kruskal-Wallis one-way ANOVA followed by Dunn’s Multiple Comparison test.
Figure 2: CS exposure inhibits the treatment effect of budesonide on OVA-induced LAR. OVA-sensitised mice (male, C57Bl/6, 18-20g) were exposed to air or cigarette smoke (b.i.d,) for 3 days. Subsequently, 1 hour after final CS challenge, mice received vehicle or budesonide (bud) (3mg kg-1, p.o.), and (1 hour later) were challenged with saline or 2% ovalbumin (i.t.). Mice were immediately placed in whole body plethysmography chambers and Penh was recorded (overnight).
Chalmers, GW, Macleod, KJ, Little, SA., et al. Thorax 57, 226-230 (2002). |
|