Print version
Search Pub Med
Quercetin-loaded microemulsion: Investigation of its Antinociceptive Properties Quercetin, a flavonoid found in many plants, fruits and vegetables is reported to have beneficial effects on human health, such as cardiovascular protection and anticancer, antinociceptive and anti-inflammatory activity1. However, clinical studies investigating different programs of administration of QU have been limited by its poor water solubility, minimal absorption in gastrointestinal tract and low bioavailability (less than 17% in rats; about 1% in men)2. Therefore, the development of quercetin-loaded nanocarrier system may be considered a promising way to exploit its therapeutic properties. This study aimed at developing a microemulsion containing quercetin and evaluating its antinociceptive effects, after oral administration, in acetic acid-induced pain test in mice. Oil-in-water microemulsion (ME) was prepared by the hot solvent diffusion method using castor oil as oily phase, and poly(ethylene glycol) (660)-12-hydroxystearate (Solutol HS15®) and soybean lecithin as surfactant and cosurfactant, respectively. To prepare the quercetin-loaded microemulsion (QU-ME), quercetin was added to the organic phase of the emulsion. The mean particle size of the system and the size distribution profile were determined by light scattering (Zetasizer/Malvern). The quercetin content and recovery were analyzed by spectroscopy using a spectrophotometer (Biomate 3) with ultraviolet detection at 375 nm. For the evaluation of antinociceptive activity, abdominal constrictions were induced by i.p. injection of acetic acid (0.6%) in male Swiss mice (25-35g), according to the procedure previously described3. Mice were treated according to the protocols (Table 1 (n=8)). The number of abdominal constrictions was cumulatively counted over a 30-min period. The statistical analysis was performed using analysis of variance followed by the Bonferoni’s post-hoc. The in vivo assays were previously approved by our University’s Ethics Committee for Animal Use. Table 1. Treatment protocols utilized in the acid acetic model in mice
a the group received only saline solution v.o b quercetin suspension in carboxymethylcellulose (0.5 % p/V) c the group received the same volume administrated in QU-ME group The microemulsions showed greater solubilizing capacities due to their large interfacial areas. The mean size of the system was around 20 nm and it was possible to encapsulate approximately 1mg/mL quercetin, which represents 2720 fold increase of quercetin solubilization in the nanocarrier by comparison with the dissolution of this drug in water. Figure 1 shows the number of abdominal constrictions evaluated during the acetic acid-induced pain test in mice. All groups treated with quercetin had a significant decrease in the number of constrictions by comparison with the control group; besides, no differences were observed with the administration of unloaded microemulsion. In the case of the quercetin suspension (QU-SUSP), a reduction in the constrictions was observed, but the increase of the dosage did not enhance the antinoceciptive effect, probably due to the limited quercetin absorption. However, in the case of QU-ME,it seems that the reduction in the constrictions number depended on the dosage; an improvement in the anti-nociceptive effect was observed when 20mg/kg was administered. 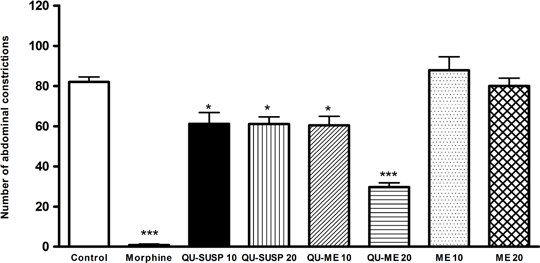
Fig 1. Number of abdominal constrictions evaluated during the acetic acid-induced pain test in mice. *p<0.05 , *** p<0.001 compared with control. Therefore, our results indicate that the microemulsion containing quercetin was successfully developed; thus, it was possible to encapsulate a great amount of the drug in the delivery system. A significantly reduction in the number of constrictions was observed when the QU-ME was used, indicating improvement in the quercetin bioavailability by comparison with with the free drug and, consequently, improvement in the anti-nociceptive effect. REFERENCES 1 Fresco, P.; Borges, F.; Diniz, C.; Marques, M.P. Medicina Research Reviews. 2006, 1-20 2 Hu, F.Q., Jiang S.P, Du Y.Z., Yuan H.,Ye Y.Q., Zeng S. International Journal of Pharmaceutics. 2006, 314, 83-89. 3 Collier H.O.J., Dinnen L.C., Johnson C.A., Schneider C. The abdominal constriction response and its suppression by analgesic drugs in mice. Br. J. Pharmacol. Chemother, 1968, 32 295–310
|

