Non-competitive antagonist binding sites in the transmembrane domain of a prokaryotic ligand-gated ion channel Introduction: There is considerable overlap between compounds that block the channels of Cys-loop receptors and the homologous prokaryotic receptors ELIC and GLIC[1][2]. In silico docking showed that these ligands were likely to bind at the M2 2’ and 6’ positions, a finding consistent with experimental evidence from Cys-loop receptors [3]. Here we explore the effects of mutations to M2 in ELIC to identify potential binding sites for one of the most potent non-competitive antagonists, picrotoxinin (PXN). Methods: Mutant receptors were created using Quikchange Site-Directed Mutagenesis (Agilent). Receptors were then expressed and tested in Xenopus laevis oocytes using two-electrode voltage clamp electrophysiology, in ND96 buffer, as previously described[1]. Concentration-inhibition curves for PXN were then measured at the EC50 for GABA. Results: EC50 values for GABA were obtained for mutant and WT receptors (Fig. 1B). The WT EC50 for GABA was 1.6 mM (pEC50 2.78 ± 0.04). 2’ and 6’mutations had no significant effect on the GABA EC50 . However mutation at the 16’ residue resulted in a significant increase in EC50 as did F16’S mutations when combined with mutations at 2’ or 6’ positions. The WT PXN IC50 was 72 μM (pIC50 4.14 ± 0.05). Mutations at the 2’ and 6’ positions did not change the PXN IC50 compared to WT. In contrast, PXN IC50 values for F16’S and F16’T mutations were significantly reduced. When F16’S was combined with T6’S or Q2’N, however, the PXN IC50 values were similar to WT Discussion: The data show that substituting the 2’ and 6’ residues in the ELIC M2 (pore lining) region have little effect on the GABA EC50, unlike mutations involving the F16’ residue, which reduce the potency of GABA. The 2’ and 6’ mutations do, however, have significant effect on the potency of the channel blocking compound PXN, but only when combined with a second M2 mutation, F16’S. These data indicate that the F16’ residue sterically prevents antagonists from descending further into the pore, as suggested by the crystal structure data, which shows the 16’ residue, occludes the extracellular end of the channel[4]. Reducing the size of this residue increases the potency for PXN, an effect that is reversed by mutation of 2’ and 6’ residues. These data indicate that 2’ and 6’ could play a role in the binding of non-competitive antagonists, but only when the aromatic ring at 16’ is removed. 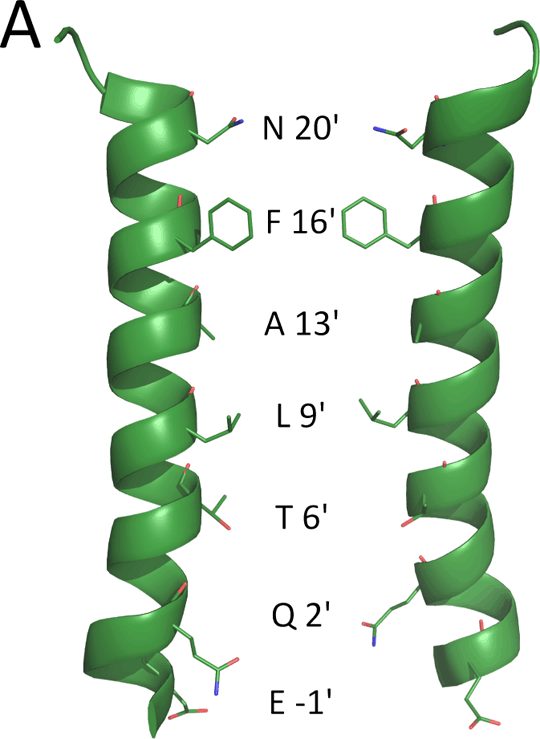 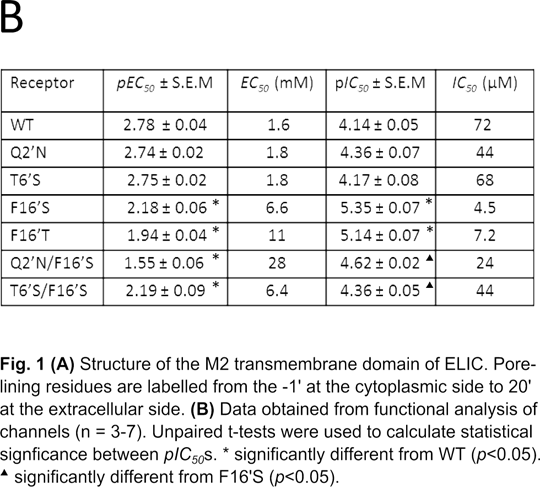
1. Alqazzaz et al. 2011, Biophys J, 101, 12. 2. Thompson et al. 2011, BPS Winter Meeting, pA2 online, 9 (3) abstract 126P. 3. Yang Z et al. 2007, J Neurochem., 103, 580. 4. Bocquet et al. 2009, Nature, 457, 111.
|

