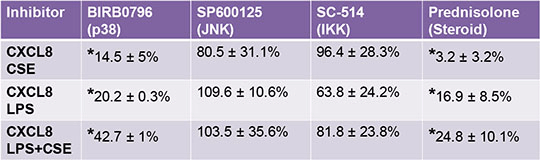
Cigarette smoke extract enhances LPS induced CXCL8 release from human whole blood Cigarette smoke is a major risk factor for a number of respiratory and cardiovascular diseases. Smoking cigarettes induces an oxidant/inflammatory challenge that is recognised to go beyond the lungs, resulting in detectable levels of oxidants and increased inflammatory cytokine expression in blood1. This is supported by clinical data associating cigarette smoking with cardiovascular diseases, such as atherosclerosis. We have previously shown that cigarette smoke activates isolated human peripheral blood mononuclear cells (PBMCs) to release CXCL8 in vitro 2. However, as far as we are aware no study has investigated the effects of cigarette smoke in an in vitro model of whole blood. We anticipate whole blood to be an improved in vitro model to study cigarette smoke induced inflammation, reflecting a complex system found in vivo. In isolated cells, the inflammatory response to cigarette smoke is enhanced by inflammatory cytokines, such as IL-1β2. In this study we investigated the effect of cigarette smoke extract (CSE), prepared as described previously2, on CXCL8 release with or without inflammatory agent lipopolysaccharide (LPS). We have gone on to partially characterise the inflammatory signalling pathways induced by CSE in this model. Whole blood was isolated from healthy donors and treated with LPS (1 µg/ml), CSE (10%) or co-stimulated of LPS+CSE (1 µg/ml and 10%). Ethical approval for the use of human cells was granted for this study by the Royal Brompton and Harefield Trust (08/H0708/69). Blood was also pre-treated for 30mins in the presence of inhibitors of p38 MAPK (BIRB0796; 0.001-1µM), JNK MAPK (SP600125; 0.3-10µM), IKK (SC-514;0.01-30µM) or the glucocorticoid receptor antagonist (Prednisolone; 0.1-100µM) (Table 1). CXCL8 was measured from plasma using ELISA as a biomarker of cell activation. Whole blood stimulated with LPS and CSE resulted in significant CXCL8 release and was significantly enhanced following co-stimulation of LPS+CSE (Control;196.3 ± 81.6, LPS;*13608 ± 1442, CSE;*4280.4 ± 2871.2, LPS+CSE;*55910.6 ±9898.8 pg/ml, data are mean ± SEM for n=15 from 8 experimental days). Statistical analysis was determined by repeated measures one-way ANOVA followed by Dunn’s multiple comparison test, *=p<0.05. Activation of whole blood with LPS+CSE was steroid sensitive and appears to be induced via activation of p38 MAPK, AP-1 pathway, but independent of NF-κB activation. This bioassay may be useful in our understanding of cigarette smoke related inflammation and in the testing of new drugs for smoking related diseases.

Table 1. Inhibition of CXCL8 release from whole blood induced by CSE (10%), LPS (1 µg/ml), and LPS+CSE (1 µg/ml and 10%) at the top concentration of inhibitors BIRB0796 (1µM), SP600125 (10µM), SC-514 (30µM), and Prednisolone (100µM) for 24h. Basal release of CXCL8 was 196.3 ± 81.6 pg/ml. Data represents mean CXCL8 release as a percentage of control (100%) ± SEM for n=3 from 1 experimental day. *=p<0.05 by one-sample T-test. 1. Paul-Clark, M.J., 2008; Molecular Medicine; 14; 238-46, 2. Walters et al., 2005; Molecular Pharmacology; 68; 1343-1353
|