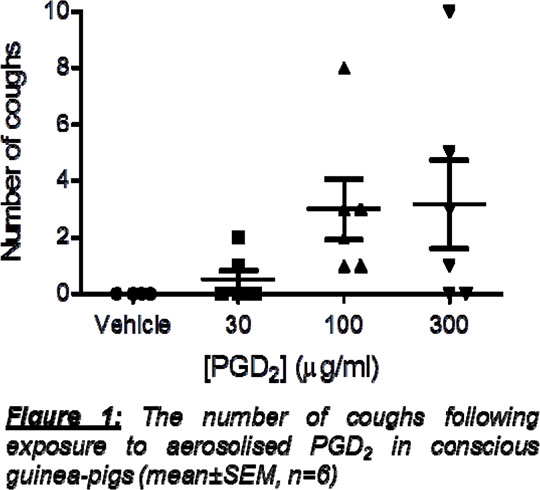
Prostaglandin D2 in airway disease and its role in the activation of sensory nerves and cough Prostaglandin D2 (PGD2) is a key endogenous mediator and the major product released from mast cells during allergic bronchospasm in man (1). Cough is a debilitating symptom of airway diseases and PGD2 has previously been shown to cause cough in cat and man (2,3). PGD2 has the highest affinity for the D-Prostanoid (DP)1 and DP2 receptors but causes bronchospasm through the TP receptor in guinea-pig and man (4,5). However, activation of the TP receptor does not cause cough (6). There is significant research investigating the role of PGD2 in inflammation and DP2 antagonists are a popular therapeutic target for airway diseases (7). However PGD2-induced sensory nerve activation and cough has not been investigated and could be an important target in the treatment of this debilitating symptom. Guinea-pig (male Dunkin-Hartley, 250-350g) conscious cough was measured using a whole body plethysmograph (Buxco). Vehicle (PBS) or PGD2 was aerosolised for 10 minutes and the number of coughs recorded. Cough occurs via the activation of airway afferents, propagation of an action potential along the vagus nerve, and a reflex motor response via the CNS. Isolated vagus nerve of guinea-pig (male, Dunkin-Hartley, 250-350g), mouse (male, 18-20g) or human (donor surplus to clinical requirement:www.iiam.org) was dissected, mounted in a grease-gap chamber and depolarisation (mV) to potential tussive agents recorded as an indicator of sensory nerve activation (8).
 
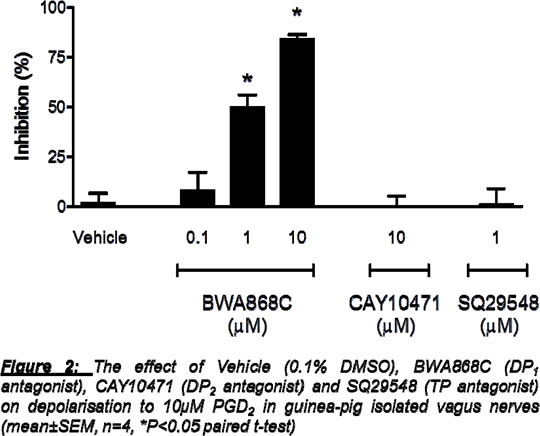
Concentration-dependant PGD2-induced cough was observed (Figure 1). In guinea-pig in vitro studies, PGD2 caused a concentration-dependent increase in depolarisation of isolated vagal nerves (0.1% Ethanol 0±0mV, 1µM 0.060±0.033mV, 3µM 0.055±0.006mV, 10µM 0.089±0.011mV, 30µM 0.118±0.031mV n=4). Vagal nerve responses to PGD2 (10µM) were significantly inhibited by BWA868C (DP1 antagonist) whereas CAY10471 (DP2 antagonist) and SQ29548 (TP antagonist) had no effect (Figure 2). A paired t-test was used to compare responses to PGD2 before and after antagonist in the same piece of nerve. Furthermore, in isolated mouse vagal nerves, Ptgdr‑/- (DP1 receptor-deficient mice), but not Gpr44-/- (DP2 receptor deficient mice) nor Tbxa2r-/- (TP receptor deficient mice), had a significantly decreased response to PGD2 compared to C57BL/6 wild types (n=4-6, C57BL/6 0.1±0.004mV, Ptgdr-/- 0.01±0.009mV, Tbxa2r-/- 0.13±0.02mV, P<0.05, Several DP2 antagonists are in development for the treatment of asthma. However, dual DP1/DP2 antagonists may be beneficial to treat cough in addition to the underlying inflammation. 1. Murray et al.,(1986) N Engl J Med 315:800-4 2. Doyle et al.,(1990) J Allergy Clin Immunol 86:924-35 3. Gardiner&Browne (1984) Prostaglandins Leukot Med 14:153-9 4. Al Jarad et al.,(1994) Br J Clin Pharmac 37:97-100 5. Beasley et al.,(1989) J Appl Physiol 66:1685-93 6. Shinagawa et al.,(2000) Br J Pharmacol 131:266-70 7. Pettipher&Whittaker.(2012) J Med Chem 55:2915−31 8. Patel et al.,(2003) Br J Pharmacol 140:261-8
|