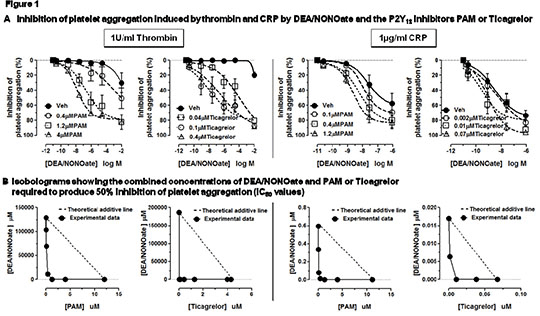
Nitric Oxide And ADP-P2Y12 Receptor Antagonists Act In Synergy To Inhibit Platelet Aggregation Activated platelets release ADP that acts on P2Y12 receptors to inhibit adenylate cyclase activity (AC). As such, when P2Y12 receptors are blocked by commonly used anti-platelet drugs such as clopidogrel, the inhibitory break on AC is lifted and the anti-platelet effects of prostacyclin and other agents that activate platelet AC are synergistically enhanced. Importantly, we have recently demonstrated that blockade of the P2Y12 receptor also enhance the anti-platelet effect of nitric oxide (NO). The aim of this study was to extend our previous observations and determine pharmacologically, using isobolographic analysis, if the positive interaction seen between NO donors and P2Y12 inhibitors on platelet aggregation constitutes a true synergy or simply an additive effect. Blood was collected by venepuncture into 0.32% trisodium citrate from 5-6 healthy volunteers. Platelets were isolated by centrifugation, washed twice in the presence of prostacyclin, and re-suspended in Tyrode’s-Hepes buffer. Washed platelets were incubated with one of two structurally distinct P2Y12 inhibitors either prasugrel active metabolite (PAM; 0.04 - 4µM) or ticagrelor (0.002 - 3µM), or their vehicle (0.5% DMSO) for 30 min. Platelets were then further treated for 1 min with the NO donor DEA/NONOate (0.02nM-10mM) or vehicle (0.01M NaOH). Platelet aggregation to thrombin (1U/ml) or collagen-related peptide (CRP; 1µg/ml in the presence of 1mg/ml fibrinogen) was then measured using 96-well plate aggregometry. Using this approach, inhibitor curves to DEA/NONOate were produced in the presence of vehicle and increasing concentrations of P2Y12 inhibitor, from which IC50 values were calculated. Isobolograms were then constructed by plotting the IC50 for DEA-NONOate against the concentration of P2Y12 inhibitor present. Both thrombin and CRP produced robust aggregation responses. CRP-induced aggregations were strongly and concentration-dependently inhibited by PAM, ticagrelor or DEA/NONOate, whereas those induced by thrombin were only weakly affected by either inhibitor individually (Figure 1A). In the presence of either PAM or ticagrelor, the sensitivity of platelets to inhibition of thrombin or CRP-induced aggregation by DEA/NONOate was enhanced (by up to 100,000 fold for thrombin) (Figure 1A). In agreement, isobolographic analysis showed that the interaction between the P2Y12 inhibitors and DEA/NONOate was strongly synergistic (isoboles curved towards the axis, away from the predicted linear line for an additive relationship) (Figure 1B).

These data confirm that activation of platelet P2Y12 receptors by secreted ADP limits the anti-platelet effect of NO, suggesting that P2Y12 activation may be an important mechanism for allowing platelet aggregation to proceed in the presence of NO. In addition, we have demonstrated that this action is strongly synergistic for thrombin and CRP-induced responses. Potentiation of the effects of endogenous NO may represent an important mechanism via which inhibitors of platelet P2Y12 receptors produce anti-thrombotic protection.
|