Print version
Search Pub Med
Modulation of Sensory Nerve Responses and Cough by Tiotropium Bromide

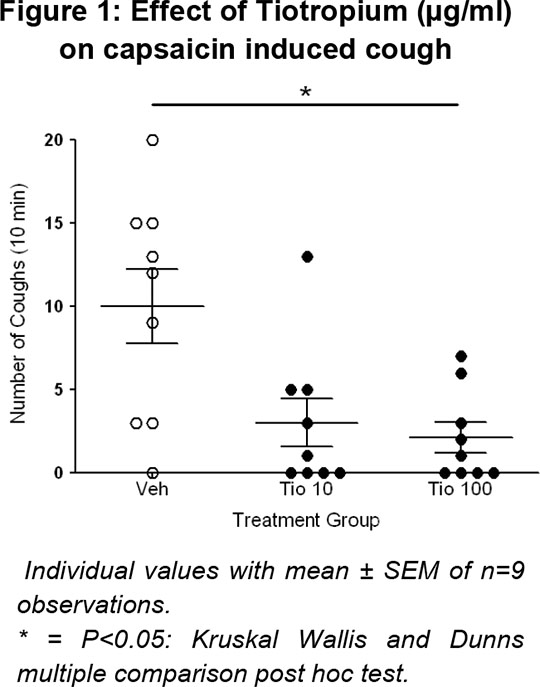
Dunkin Hartley GPs (Male, 300-350g) were exposed to aerosolised vehicle (0.1% DMSO in saline for 10 minutes) or Tio (10 and 100 µg ml-1) in a Perspex box. Fifty minutes later they were challenge with aerosolised capsaicin (60 µM, a TRPV1 ion channel agonist). The results showed that Tio significantly attenuated the cough response (Figure 1).

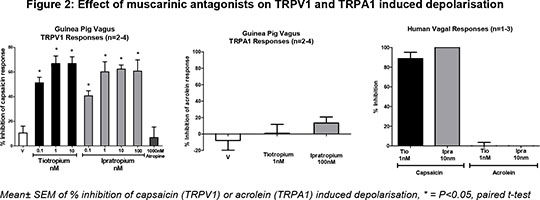
To assess the effect of Tio, and other muscarinic antagonists, on isolated sensory nerve activity we employed an in vitro vagal depolarisation system4. This system has the advantage that it is not affected by changes in airway tone and by using human tissue we can obtain translational data. Sensory nerves were activated using capsaicin (1 µM) and a TRPA1 ion channel agonist acrolein (300 µM) to induced depolarisation. Tio, and ipratropium bromide (a short acting muscarinic antagonist from which the structure of Tio is derived 5), caused a concentration dependant inhibition of capsaicin responses in GP and human vagus, whilst atropine did not (Figure 2). Furthermore the inhibition appeared to be specific to the TRPV1 channel as neither compound altered responses to acrolein. Finally to confirm that Tio is modulating TRPV1 responses in airway sensory nerves, we isolated primary airway jugular ganglia cells (using a retrograde labelling technique6). We used capsaicin (0.1 µM) and acrolein (10 µM) induced calcium movement to show that Tio, (0.1 and 1nM) but not atropine, significantly inhibited capsaicin but not acrolein induced responses. (0.1nM Tio caused 77.02±6.6% inhibition of the capsaicin response, 1nM caused 94.4±3.1% inhibition, N=2-3 animals, 4-12 cells, p<0.05, paired t-test). In summary, our data suggests that Tio inhibits TRPV1 ion channel activity via a mechanism unrelated to its anti-cholinergic activity. This activity is not via a general inhibition of sensory nerve activity as TRPA1 mediated responses were not affected. Furthermore, we suggest that some of the clinical benefit associated with taking Tio could be explained because of its blockade of TRPV1 responses. 1. Barnes PJ.(2000)Chest.;117:63S-6 2. Dicpinigaitis PV et al.(2008).Lung. 186:369-74 3. Bouyssou T. et al.(2009).AJRCCM;179:A4558. 4. Birrell MA et al.(2009).AJRCCM;180:1042-7. 5. Barnes PJ et al.(1995)Life Sciences,11/12:853-859 6. Undem BJ, et al (2004). J.Physiol.556:905-17.
|