Print version
Search Pub Med
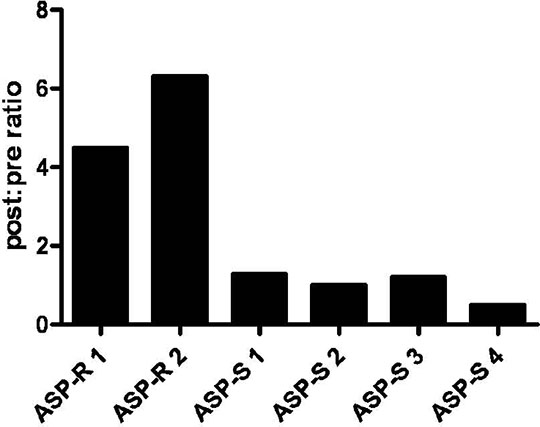
Identification of a Novel Biomarker of Aspirin Resistance Introduction: Aspirin (acetylsalicylic acid) is integral to the secondary prevention of cardiovascular events, yet a proportion of patients fail to appropriately inhibit platelet function in a heterogeneous phenomenon known as aspirin resistance. Light transmission aggregometry (LTA) is the current ‘gold standard’ for the identification of aspirin resistance but is not suitable for clinical application. Here we present data on the prevalence of aspirin resistance in a healthy population and the identification of a novel proteomic biomarker of aspirin resistance. Methods and Key Findings: 93 healthy subjects, on no regular medication, received aspirin 300mg once daily for 28 days. Aspirin resistance was defined as >20% aggregation of platelet-rich plasma (PRP) in response to 1.6mM by LTA (Payton 600 dual channel aggregometer) and urinary 11-dehydrothromboxane B2 >67.2ng/mmol of creatinine by ELISA (Cayman Chemical Co.). Across the population, mean platelet aggregation and levels of urinary 11-dehydrothromboxane B2 were suppressed following aspirin therapy (p<0.001), However, in 2 subjects neither AA-induced aggregation nor urinary 11-dehydrothromboxane B2 was effectively suppressed by aspirin treatment despite measurable plasma salicylate levels in these subjects. The platelet proteome of these two subjects was compared to that of four aspirin-sensitive subjects using TMTsixplex® (Thermo Scientific) labelling and gel electrophoresis and liquid chromatography - mass spectrometry/mass spectrometry (GeLC-MS/MS). No detectable differences in the platelet proteome at baseline were identified between aspirin-sensitive and aspirin-resistant subjects, but following aspirin therapy a marked increase was seen in platelet expression of glycoprotein IIIa (GPIIIa) in the aspirin-resistant but not aspirin-sensitive subjects. These findings were confirmed by Western blotting which found approximately a four-fold increase in GPIIIa expression when compared to vinculin (p<0.01) (antibodies from Santa Cruz Biotechnology) (Figure 1).

Figure 1 Comparison of the expression of GPIIIa in aspirin resistant (ASP-R) and aspirin sensitive (ASP-S) subjects pre and post aspirin therapy (p<0.01 by unpaired t test). Using GeLC-MS/MS, an Absolute QUAntification (AQUA) strategy using single reference point internal standards was used to develop an assay to measure GPIIIa expression and its isoforms in platelets. In-gel AspN digestion of platelet lysates was performed on samples from 29 healthy aspirin-naïve subjects. Mean expression of GPIIIa was 8.80 pg/µg (SEM = 1.60 pg/µg) total protein. Of the three known isoforms of GPIIIa (A, B and C), only isoform A was detectable. Conclusion: Aspirin resistance is uncommon in a healthy population and appears to be associated with an increase in platelet GPIIIa expression in response to aspirin exposure. We have developed a mass spectrometry assay which will enable this association to now be explored further in the clinical setting.
|