Print version
Search Pub Med
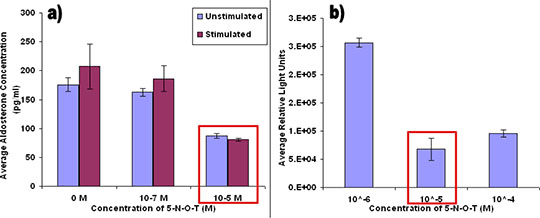
Investigation of zonal regulation of steroidogenisis in the human adrenal with emphasis on GPR61, a potential candidate regulator of aldosterone secretion Background: Primary Hyperaldosteronism (PH) or Conn’s syndrome is the commonest curable cause of hypertension, affecting 5 to 15% of patients with the disease. In some patients with PH, benign tumours which are formed in the adrenal gland(s) secrete aldosterone, the hormone which stimulates re-absorption of sodium ions in the distal nephron. In a microarray comparison of aldosteronomas with their paired normal adrenals where 19,594 genes were screened, a cAMP coupled biogenic amine-like receptor; GPR61 was amongst the most significantly upregulated genes, 8-fold in tumours1. Since GPR61 activation in vitro stimulates cAMP production, the major intracellular stimulus to aldosterone production, we hypothesise that GPR61 is a regulator of aldosterone which could be a novel therapeutic target for treatment of hypertension or other conditions associated with aldosterone regulation. Aims: To provide evidence of the role for GPR61, by investigating its expression anatomically and functionally within the human adrenal. Methods: Fresh adrenal pairs after adrenalectomy from patients with PH were collected. GPR61 expression was quantified by qPCR in 44 pairs of normal adrenals with their paired aldosteronomas. Tissue distribution of GPR61 was also measured in both human and mice. Translation to protein was assessed by Western blotting, and its distribution between adrenal zones assessed by immunohistochemistry (IHC) and confocal microscopy. Preliminary functional evidence of GPR61 regulation of aldosterone secretion was sought using its surrogate low-affinity 5-HT-like ligand, 5-(nonyloxy)-tryptamine (5-N-O-T) in primary adrenal cell cultures. Endogenous activity for GPR61 was investigated by screening 5-N-O-T and other compounds through a β-arrestin recruitment assay. Results: qPCR replicated the microarray result, showing a 5.87-fold higher expression (where ddCT expression difference was -2.56) in adenomas versus the normal adrenal, but with >100-fold variation between patients. Intriguingly, GPR61 expression on mRNA level varied from protein level as shown in Western blots. IHC of GPR61 showed the majority of staining to be in ZG, the site of aldosterone synthesis. 5-N-O-T at 10-5M, the known Kd for cloned GPR612, down regulates aldosterone secretion of primary adrenal cells. In the presence of angiotensin II, the physiological regulator of aldosterone, the down regulation of aldosterone was amplified (n=5; P<0.004). The 5-N-O-T effect was also observed in eukaryotic cells that were expressing GPR61 and involved in the B-arrestin pathway as can be seen in figure 1.

Figure 1: (a) Radioimmunoassay of aldosterone measurements shows that at concentration of 10-5M 5-N-O-T, aldosterone is down regulated (n=5). The blue bars represent the unstimulated (control) measurements, whereas the red represents, the stimulated or treated with angiotensin II measurements. A similar result is shown in figure 1 (b), where a β-arrestin recruitment assay shows GPR61 binding to 5-N-O-T at 10-5M concentration. Conclusion: Structurally, GPR61 may be playing a role in the regulation of aldosterone secretion from ZG, and in the development of aldosterone-secreting tumours. This could provide therapeutic insights to diagnosing hyperaldosteronsism associated with resistant hypertensives.
References: 1. Azizan, E. A. B., Lam, B. Y. H., Newhouse, S. J., Zhou, J., Kuc, R. E., Clarke, J., Happerfield, L., Marker, A., Hoffman, G. J., Brown, M. J. 2012. Microarray, qPCR, and KCNJ5 Sequencing of Aldosterone-Producing Adenomas Reveal Differences in Genotype and Phenotype between Zona Glomerulosa- and Zona Fasciculata-Like Tumors. J Clin Endocrinol Metab. 97(5):E819-29. 2. Toyooka, M., Tujii, T., Takeda, S. 2009. The N-terminal domain of GPR61, an orphan G-protein-coupled receptor, is essential for its constitutive activity. J. Neurosci. Res. 87, pp. 1329–1333.
|