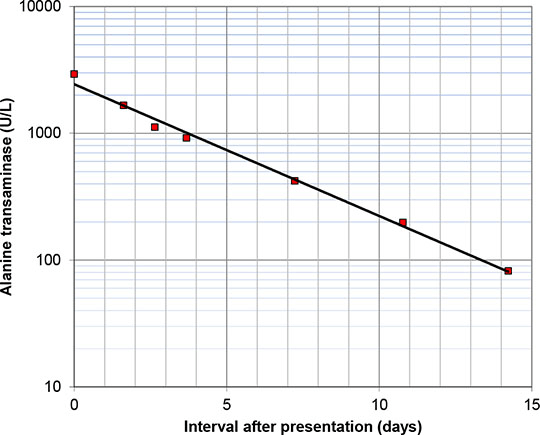
Modified-Release Metformin Associated With Drug-Induced Hepatitis Case report A 71-year-old man was commenced on metformin 500 mg twice daily for type 2 diabetes. Past medical history included type 2 diabetes and hypertension, and regular medications were aspirin 75 mg daily, ramipril 2.5 mg daily and rosuvastatin 10 mg nocte. He developed progressive nausea and abdominal discomfort. Treatment was discontinued, and his symptoms resolved over the next two weeks. Metformin modified release 500 mg twice daily was introduced. Again he developed progressive nausea, abdominal pain and lethargy, and he was referred to hospital. Investigations showed alanine transaminase 2931 U/L (reference range 0-45 U/L), bilirubin 109 µmol/L (0-21 µmol/L), alkaline phosphatase 183 U/L (30-130 U/L), prothrombin time 12 seconds (9.6 to 12.3 seconds); electrolytes were normal, and ultrasound appearances of liver and biliary tree were normal. Metformin and rosuvastatin were discontinued, and patient symptoms and liver biochemistry tests progressively improved (Figure 1); rosuvastatin was later reintroduced and liver biochemistry remained normal.

Figure 1. Serum alanine transaminase at various intervals after discontinuation of metformin. Discussion Drug-induced hepatitis is an uncommon but recognised adverse effect of metformin. Up to July 2012, the Yellow Card scheme in the United Kingdom had received 2383 reports of adverse effects of metformin as sole suspected agent, including 92 fatal reports; 11 (0.5%) of these were related to acute liver injury, including 2 reports (0.1%) of acute liver failure [1]. Causality in this case was based on (1) resolution of symptoms after metformin cessation, with recurrence after drug reintroduced, (2) a close temporal relationship between drug administration and development of symptoms, (3) normalisation of liver biochemistry after metformin cessation, and (4) the lack of a plausible alternative explanation. We cannot exclude the possibility that rosuvastatin might have contributed to the extent of metformin-induced liver injury, however rosuvastatin and other prior medications had not been associated with any liver derangement in this patient. Conclusions Drug-induced hepatitis is a rare but recognised adverse effect of metformin therapy, and should be considered in patients that develop unexplained abdominal symptoms.
Reference 1. Medicines and Healthcare Products Regulatory Agency. Dug Analysis Print Metformin. http://www.mhra.gov.uk/home/groups/public/documents/sentineldocuments/dap_1345639442350.pdf
|