Print version
Search Pub Med
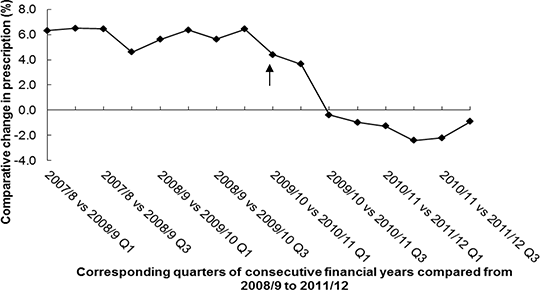
Impact of the Medicines and Healthcare products Regulatory Agency’ s safety update on primary care prescribing of quinine in the UK INTRODUCTION: Quinine is commonly prescribed for leg cramps in the UK, although clinical trials have suggested limited benefit and it is associated with serious toxicity, including impaired hearing and vision, cardiac arrhythmia and death, especially after overdose. As a result, the USA FDA ordered a withdrawal of quinine products being used off-licence for muscular cramps (1, 2). Because of concerns about the benefit-risk balance for quinine, the UK Medicines and Healthcare products Regulatory Agency (MHRA) issued a safety update in June 2010 advising that the use of quinine for leg cramps should be limited (3). This study was performed to analyse the impact of this safety update on national and local prescribing trends. METHOD: Data on quinine prescribing in England and in north-east Primary Care Trusts (PCTs) were obtained from the Prescribing Analysis and Cost database. Joinpoint regression was used to test for changes in trends employing SEER*Stat software version 3.5.3 (4). RESULTS: Quinine prescribing in England has significantly changed from a mean annual growth of 6.0 % to a decline of 0.6 % following the MHRA safety update (Estimated joinpoint position -2nd Quarter of 2010/11 [95% CI 1st Quarter 2009/10 to 4th Quarter 2010/11]; difference in slopes -0.0401 [95% CI -0.0672 to -0.0130], p value =0.0111). The decline in quinine prescribing following the update was most marked in the second quarter of 2011/12; since then, a reversal in this trend has been observed (Estimated joinpoint position -4th Quarter 2010/11 [95% CI 2nd Quarter 2010/11 to 2nd Quarter 2011/12]; difference in slopes 1.7984 [95% CI 0.4149 to 2.5466]; p value =0.0343) (Figure 1). Figure 1: Comparative quarterly changes in the volume of quinine prescribed

Changes in prescribing volume of quinine during corresponding quarters from 2008/9 to 2011/12. MHRA quinine safety update publication (↑). The effect of the update on prescribing trends differed amongst PCTs. The volume of prescribing in Hartlepool declined from 26.3 to 7.6 items/1000 patients following the institution of a GP led initiative (Estimated joinpoint position – 1st Quarter 2010/11 [95% CI 3rd Quarter 2009/10 to 2nd Quarter 2010/11]; difference in slopes pre- and post-MHRA update -0.6337 [95% CI -0.905 to -0.487], p value =0.0006). This involved reviewing quinine use with the aim of removing it from repeat prescriptions by providing users with information on the limited efficacy of quinine in leg cramps and the associated potential toxicities, as well as encouraging the use of other safer treatment modalities. In contrast, prescribing has remained stable in many PCTs following the update but continued to increase in others. CONCLUSION: Quinine prescribing has stabilised following the safety update but early indications suggest that this may be a short term effect. Wider adoption of more comprehensive initiatives as used in Hartlepool PCT may reduce quinine prescribing further.
ReferenceS: (1) Benowitz NL. In: Poisoning and Overdose, Olson KR, editor. 6th ed. San Francisco, California: McGraw - Hill; 2011;358-359. (2) Food and Drug Administration, Department of Health and Human Services. Drug products containing quinine; enforcement action dates. Federal Register 2006(71):75557–75560 (3) Tilstone C. Drug Safety Update 2010;3:3. (4) Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates Stat Med 2000;19:335-351(correction: 2001;20:655).
|