162P Queen Elizabeth II Conference Centre London
BPS Winter Meeting 2012 |
Effects of the Medicines and Healthcare products Regulatory Agency’ s safety update on the frequency and characteristics of quinine toxicity in the UK
P Acheampong1, DJ Lupton2, G Cooper3, B Khazaeli1, S White1, MT May4, SHL Thomas1,5. 1Newcastle upon Tyne NHS Foundation Trust / Regional Drug and Therapeutics Centre, NE2 4HH, Newcastle upon Tyne, UK, 2National Poisons Information Service, EH16 4SA, Edinburgh, UK, 3National Poisons Information Service, CF64 2XX, Cardiff, UK, 4School of Social and Community Medicine, University of Bristol, BS8 2PS, Bristol, UK, 5Wolfson Unit of Clinical Pharmacology, Institute of Cellular Medicine, Newcastle University, NE2 4HH, Newcastle upon Tyne, UK
INTRODUCTION: Quinine is commonly prescribed for leg cramps although available evidence indicates limited clinical benefit, and substantial placebo effect has been observed in clinical trials (1, 2). Quinine may cause severe toxic effects especially in overdose (3). Because of concerns about the benefit-risk balance for quinine use in leg cramps, the UK Medicines and Healthcare products Regulatory Agency (MHRA) issued a safety update in June 2010 advising restricted use (4). In a separate report, we have demonstrated that this has led to a reversal in growth of quinine prescribing. In this study, the impact on quinine toxicity, especially in overdose, is examined.
METHOD:
Data on telephone enquiries and accesses to the Toxbase® website relating to quinine from 2008/9 to 2011/12 were obtained from the National Poisons Information Service (NPIS). Characteristics of quinine toxicity from telephone enquiries and the frequency of Toxbase® quinine user sessions were analysed. Pearson’s correlation and joinpoint regression tests were used to test for associations and changes in trends respectively.
RESULTS: Before the MHRA update, Toxbase® quinine accesses were increasing but this has since shown a reversal in trend (difference in slopes -19.76 [95% CI -39.28 to -9.20], p value = 0.0575). These changes correlate with changes in the volume of quinine prescribing in England (correlation coefficient=0.5594; p value=0.0103) (Figure 1).
Figure 1: Trends of Toxbase® enquiries and quinine prescribing
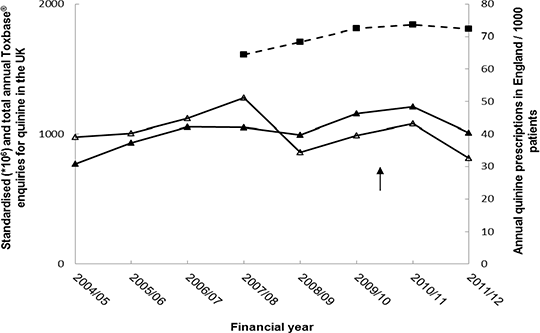

Quinine prescribing in England (▪). Total (▴) and standardised [ratio of total quinine to total product] (∆) annual Toxbase® quinine accesses. MHRA quinine safety update publication (↑).
In the fiscal years 2008/9 to 2011/12, NPIS received 510 telephone enquiries involving quinine. Of these, 259 (51%) related to cases of accidental use or therapeutic error who ingested doses commonly between 0.2 and 0.9g, and remained mostly asymptomatic. Deliberate self-harm accounted for nearly 40% of all cases with a median ingested dose (range) of 4.4 (0.9–19.0)g and was responsible for most symptomatic reports. The incidences of deliberate self-harm and serious toxic features associated with quinine overdose are shown in Table 1. Other serious clinical features over the 4 year period included acidosis (28), stupor or coma (18), hypoglycaemia (8), convulsions (4) and death (2). Telephone enquiry numbers were not large enough for detailed statistical analysis of time trends.
Table 1: Characteristics of telephone enquiries to NPIS relating to quinine
| |
2008/9 |
2009/10 |
2010/11 |
2011/12 |
| Self-harm, n (%) |
53(39%) |
63(43%) |
34(32%) |
46(38%) |
| Cardiac toxicity, n (%)* |
18(13%) |
33(22%) |
29(28%) |
26(21%) |
| Visual impairment, n (%) |
17(13%) |
26(18%) |
16(15%) |
14(11%) |
| Hearing impairment, n (%) |
10(7%) |
6(4%) |
3(3%) |
13(11%) |
| Cardiac toxicity, visual or hearing impairment, n (%) |
45(33%) |
65(44%) |
48(46%) |
53(43%) |
| Total number of cases (n) |
136 |
147 |
105 |
122 |
*Defined as bundle branch block, bradycardia, tachyarrhythmia, QT prolongation and/or cardiac arrests.
CONCLUSION: The MHRA safety update and subsequent reductions in prescribing have been associated with reduced Toxbase® accesses. Reductions in telephone enquiries, especially those involving serious toxicity, cannot be demonstrated due to low numbers. Further gains may be realised by encouraging more substantial reductions in quinine prescribing.
ReferenceS:
(1) Man-Son-Hing M, Wells G, Lau A. Quinine for nocturnal leg cramps: a meta-analysis including unpublished data. Journal of General Internal Medicine 1998;13:600-606.
(2) Diener HC, Dethlefsen U, Dethlefsen-Gruber S, Verbeek P. Effectiveness of quinine in treating muscle cramps: a double-blind, placebo-controlled, parallel-group, multicentre trial. Int J Clin Pract 2002;56:243-246.
(3) Benowitz NL. In: Poisoning and Overdose, Olson KR, editor. 6th ed. San Francisco, California: McGraw-Hill; 2011;358-359.
(4) Tilstone C. Drug Safety Update 2010;3:3.
|