Print version
Search Pub Med
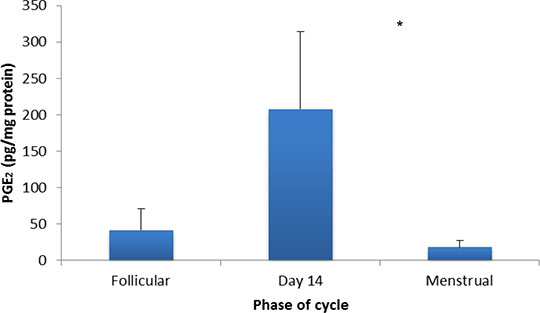
Functional Prostaglandin E Receptors In Isolated Human And Rat Myometrium Prostaglandin (PG) E2 acts through its receptors (EP1-4) to promote angiogenesis, cell proliferation and to modulate uterine activity. Whilst it facilitates normal reproductive processes, such as sperm transport, ovulation and blastocyst implantation, aberrant PGE2 output has been reported to reduce fercundity in women (Dixon et al., 2009) and in mice (Tilley et al., 1999). To assess the importance of PGE2 in uterine motility, ex vivo PG profiles and in vitro responses to PGE2 were characterised mid-cycle in non-gravid human myometrium. EP-mediated effects were also examined in the rat to determine its suitability as a model of human reproductive disorders. Uterine samples were obtained from consenting pre-menopausal women undergoing hysterectomy for benign disorders (n=6) and from Lister Hooded rats (n=6). Studies were carried out in accordance with the Local Regional Ethics Committees and the Animals Scientific Procedures Act (1996). For contraction recordings, myometrial strips were mounted in immersion baths under physiological conditions and attached to isometric force transducers. Following tissue equilibration, vehicle and concentration-effect curves (10-9M to 10-5M) were constructed for PGE2, butaprost (an EP2 agonist; Gardiner, 1986), L-902688 (an EP4 agonist; Billot et al., 2003), ONO-D1-004 (an EP1 agonist; Oka et al., 2003) and sulprostone (an EP3/1 agonist; Schaaf et al., 1981). Stock solutions were prepared using ethanol. To assess endogenous PGs (n=9), human myometrial biopsies were extracted and quantified using liquid chromatography coupled with ionisation mass spectrometry (Nicolaou & Masoodi, 2006). Results were expressed as means ± S.E.M and analysed using ANOVA with Bonferroni’s post-hoc test. The results show that myometrial PGE2 output was most abundant around ovulation relative to menses (p<0.05; Figure 1). In functional studies, compared to vehicle controls, PGE2 produced bell-shaped responses with predominant utero-relaxant effects in the human (p<0.001) and excitatory effects in the rat (p<0.01). Exposure to butaprost evoked pronounced concentration-dependent inhibition of activity in human myometrial strips (p<0.001), whereas L-902688 stimulated activity at 10-5M in the rat (p<0.01). Sulprostone further enhanced the amplitude and frequency of contractions in human (p<0.01) and rat tissues (p<0.001); ONO-D1-004, however, had little effect. 
Figure 1: Ex vivo PGE2 output in isolated myometrium obtained from non-gravid women with typical menstrual cycles (n=9); *p<0.05 for day 14 compared to the menstrual phase. These findings indicate that the profound in vitro inhibitory effect of PGE2 in human myometrium was mediated by the EP2 subtype with some excitation achieved through a complement of EP3 receptors. Utero-quiescence during the peri-ovulatory peak in PGE2 may facilitate pregnancy. Even so, since PGE2 in the rat was conversely uterotonic, it may not be easily transposed as a model of human uterine pathologies.
References: Billot et al., 2003 Bioorg Med Chem Lett, 13, 1129-1132. Dixon et al., 2009 Biol Reprod, 80, 665-673. Gardiner, 1986 Brit J Pharmacol, 87, 45-45. Oka et al. (2003). Brain Res 968: 256-262. Nicolaou & Masoodi (2006). Rapid Commun Mass Spectrom 20: 3023-3029. Schaaf et al. (1981). J Med Chem 24: 1353-1359. Tilley et al., 1999 J Clin Invest, 103, 1539-1545.
|