Print version
Search Pub Med
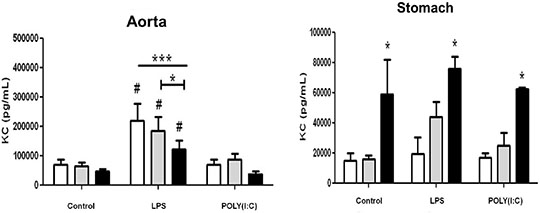
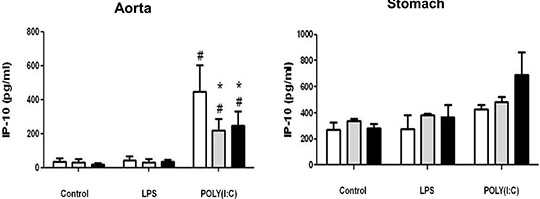
Differential effects of cyclo-oxygenase (COX)-1 and COX-2 in myeloid differentiation primary response gene (88) (MyD88) and TIR-domain-containing adapter-inducing interferon-β (TRIF) cytokine release in aorta versus stomach Introduction Cyclo-oxygenase (COX) is expressed in two isoforms. COX-1 is expressed constitutively whilst COX-2 is induced at sites of inflammation. Nonsteroid anti-inflammatory drugs (NSAIDs) owe their therapeutic utility and their side effects to inhibition of COX. Side effects associated with some NSAIDs include gastric ulceration and increased risk of cardiovascular events. NSAIDs that selectively inhibit COX-2 were introduced as a new class a decade ago, in the belief they should have reduced gastrointestinal effects. However, COX-2 selective NSAIDs, as with non-selective older drugs, retain a cardiovascular risk. The relationship between COX-1, COX-2 and inflammation in vessels and the gut remains the subject of investigation. In this study we have used tissue culture models with mouse vessel (aorta) versus gastric tissue (stomach) to investigate how inflammatory cytokines induced by bacterial or viral mimetics are affected by COX-1 or COX-2 gene deletion. The mouse CXCL-8 homologue KC was measured as a marker of the myeloid differentiation primary response gene (88) (MyD88) pathway activated by lipopolysaccharide (LPS) and Interferon gamma Inducible Protein-10 (IP-10) as a marker of the TIR-domain-containing adapter-inducing interferon-β (TRIF) pathway activated by polyinosinic:polycytidylic acid (POLY(I:C)). Methods Aortae and stomach tissue from wild type, COX-1-/- and COX-2-/- knockout mice (n=3-8) were incubated in vitro for 48 hours with LPS or POLY(I:C). KC and IP-10 release were measured in conditioned media by commercial ELISA, according to manufacturer’s instructions. Data were analysed by 2-way ANOVA using Bonferroni post test and Dunnett’s post test. Results Aorta or stomach release KC (Figure 1) and IP-10 (Figure 2) in tissue culture. In aorta, but not stomach, KC was increased by LPS, but not POLY(I:C) and IP-10 was increased by POLY(I:C) but not LPS. KC release induced by LPS or IP-10 release induced by POLY(I:C) from aorta was reduced in tissue from COX-2-/- mice. By contrast KC was increased in all conditions from the stomach of COX-2-/- mice.

Figure 1: Release of KC from vascular and stomach tissue from wild type, COX-1-/-or COX-2-/- mice in tissue culture in response to LPS (10µg/ml) or POLY(I:C) (10µg/ml). Data is mean ± S.E.M for tissue from n=3-8 animals analyzed by ANOVA. *P<0.05 genotypes compared to wild type (WT) using Bonferroni posttest: #p<0.05 treatment compared to respective control using Dunnett's posttest.

Figure 2: IP10 release from vascular and stomach tissue from wild type, COX-1-/- or COX-2-/- mice in tissue culture in response to LPS (10µg/ml) or POLY(I:C) (10µg/ml). Data is mean ± S.E.M for tissue from n=3-8 animals analyzed by ANOVA. *P<0.05 genotypes compared to wild type (WT) using Bonferroni posttest: #p<0.05 treatment compared to respective control using Dunnett's posttest. Conclusion COX activity regulates cytokine release differently in vessels compared to the stomach. Further research on this may improve our understanding of the regulation by COXs of cytokine release in these tissues.
|