194P Queen Elizabeth II Conference Centre London
BPS Winter Meeting 2012 |
Pharmacology of IFNα and IFNλ in hepatocytes and human lung microvascular endothelial cells; relevance to pulmonary inflammation secondary to Hepatitis C therapy
PM George1, JA Price2, H Gashaw1, N Galloway-Phillipps1, JA Mitchell1. 1Imperial College, London, UK, 2Lifetechnologies (TM), Paisley, UK
Introduction: Chronic Hepatitis C viral (HCV) infection affects approximately 170 million individuals worldwide. Interferon-α (IFNα) has been the cornerstone of HCV therapy for many years. However, in some individuals, IFNα can cause pulmonary toxicity in the form of pneumonitis and pulmonary vascular disease. Interferon-λ (IFNλ) is currently in Phase III clinical trials for the treatment of HCV and appears to possess equivalent anti-viral activity to IFNα. We wished to profile the effects of IFNα and IFNλ in hepatocytes and human lung microvascular endothelial cells (HMVECs) to enable a comparative understanding of the potential pulmonary side effect profile of these two different IFN therapies. We measured interferon-gamma inducible protein-10 (IP10) as a marker of cellular activation.
Methods: Cryopreserved human hepatocytes were purchased from LifetechnologiesTM, UK and plated on 96 well plates at a seeding density of 5,200 cells per well in hepatocyte plating media (LifetechnologiesTM, UK), which was replaced at 4hours by Williams Medium E containing cell maintenance supplement. HMVECs were purchased from Lonza, UK and were seeded at 8,000 cells per well on 96 well plates in HMVEC media (Lonza, UK). Hepatocytes were then serum deprived for 24 hours and both cell types were subsequently treated for 24 hrs with IFNs with and without TNFα (10ng/ml). IP10 levels were measured in the supernatant using ELISA (R&D, UK).
Results: In the presence of TNFα, both hepatocytes and HMVECs were sensitive to the effects of IFNα, releasing IP10 in a concentration dependent manner (Fig 1a). There was no significant difference in the response of the two cell types to IFNα (Fig 1a). Conversely, hepatocytes released significantly greater levels of IP10 than HMVECs in response to IFNλ at all concentrations tested (p<0.05) (Fig. 1b).
Discussion: Both hepatocytes and HMVECs are activated by IFNα and this effect is augmented by TNFα. However, while hepatocytes sense IFNλ avidly to release IP10, HMVECs are insensitive to IFNλ regardless of the presence of TNFα. This effect is likely to be a reflection of the relative exclusivity of the IFNλ receptor, which is known to be present only on epithelial surfaces and hepatocytes as opposed to the receptor for IFNα, which is ubiquitously expressed. These data suggest that IFNλ
could be an antiviral therapy that spares lung vascular cells and may therefore have a propensity to a reduction in pulmonary side effects as compared to IFNα.
Conclusion: IFNα and IFNλ differ in their cell selectivity and this may have relevance to their potential side effect profiles when utilised therapeutically.
Figures:

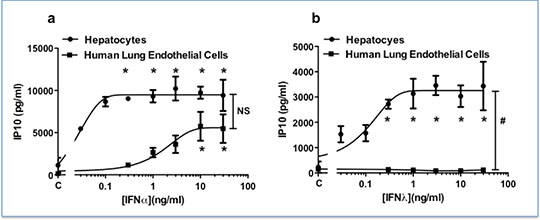
Fig 1: Cell selective effects of IFNα (a) and IFNλ (b) on hepatocytes (n=3) and human lung microvascular endothelial cells (HMVECs) (n=4) in the presence of TNFα (10ng/ml). Without TNFα, Emax for IP10 release in IFNα treated cells was 10,705.9pg/ml for hepatocytes and 243.8pg/ml for HMVECs. Without TNFα, the Emax for IP10 release in IFNλ treated cells was 2942.5pg/ml for hepatocytes and 7.80pg/ml for HMVECs. Data shown as mean ± SEM and was analysed using two-way ANOVA (# p<0.05) followed by Bonferroni post-test (* p<0.05).
|