Carbamazepine Hypersensitivity: Linking Metabolism to the Immune Response
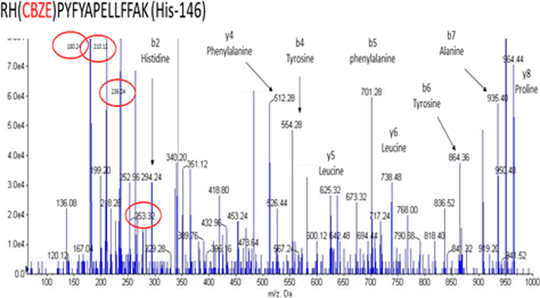
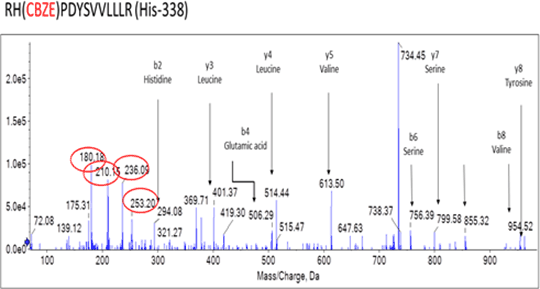
Carbamazepine (CBZ) is an effective antiepileptic drug. Although generally well tolerated it can cause cutaneous adverse drug reactions (ADRs) in up to 10% of patients (1), ranging from mild maculopapular exanthema (MPE) to Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN). The clinical features of CBZ hypersensitivity suggest an immune aetiology. The discovery of strong genetic associations between specific human leukocyte antigen (HLA) alleles, HLA-A*3101 and HLA-B*1502, and CBZ hypersensitivity support this theory. It has been proposed that CBZ is metabolised into reactive metabolites that form haptens with cellular proteins, which are then presented by specific HLA alleles and activate the immune system in susceptible individuals (2). The aim of my research is to investigate genetic variation in the metabolism of CBZ, to identify CBZ haptens and to delineate the mechanism by which immune stimulation occurs. CBZ 10,11-epoxide (CBZ-E) is the major stable metabolite of CBZ and has therapeutic activity. CBZ-E was incubated with human serum albumin (HSA) over a range of concentrations (0.1:1 to 25:1) and time points (1h, 4h, 24h and 48h). Mass spectrometry was used to analyse the samples and two CBZ-E and HSA adducts were identified at histidine residues 146 and 338 (fig 1). These adducts are novel and have never been identified before.  
Further in-vitro work will focus on incubation of CBZ with human liver microsomes and HSA. In future, plasma samples will be obtained from healthy volunteers, patients newly starting CBZ and patients on maintenance CBZ therapy. Plasma levels of CBZ, CBZ metabolites and CBZ-protein adducts will be determined and incorporated into a population PK model. This model will also include genetic variation in drug metabolism enzymes, detoxification pathways and transporter proteins. CBZ-protein adducts identified in-vivo will be utilised in the in-vitro lymphocyte transformation test to determine their ability to stimulate the immune response and transcriptome analysis will be undertaken to determine which pathways are modulated on exposure to CBZ (metabolites). The aim of this research is to identify risk factors for CBZ hypersensitivity and develop new biomarkers that enable clinicians to recognise patients at risk of hypersensitivity reactions. 1. Marson et al.(2007) The Lancet 369: 1000-1015 2. Yip et al. (2012) Journal of Clinical Pharmacology and Therapeutics 92: 757-765
|