Drug Withdrawals Because of Deaths After Marketing: a Systematic Review
Post-marketing withdrawal of a drug because of deaths can be occasioned by evidence obtained from anecdotal reports, observational studies, clinical trials, or systematic reviews. While the links between some drugs and deaths have been established, the mechanisms by which some other withdrawn medications caused deaths have not been clearly shown. We have identified medicinal products that have been withdrawn as a result of deaths, using published evidence. This work builds on a previous analysis of 284 medications that have been withdrawn or had their labels changed following reports of adverse drug reactions, including deaths [1]. We searched the WHO’s Consolidated List of Products, the European Medicines Agency’s database of withdrawn drugs, the Medicine and Healthcare products Regulatory Agency’s database, the Food and Drug Administration’s database, MEDLINE, Google Scholar, and Meyler ’ s Side Effects of Drugs and the Side Effects of Drugs Annuals 1 – 34. We found 39 drugs that were withdrawn following reports of deaths. At least 50% were withdrawn in more than one country, but only 10 were withdrawn worldwide. The highest number of withdrawals occurred during the 1990s (31%). The evidence came from clinical trials data in eight cases, but most came from case reports (n=28; 72%). Respiratory depression, hepatotoxicity, and cardiotoxicity together accounted for over 50% of withdrawals. Two drugs were withdrawn because of deaths from interactions with other medications. There were differences in the patterns of withdrawal in different countries. For example, in the UK troglitazone was withdrawn because of deaths due to liver damage, but in the USA the label was changed to require more extensive monitoring of liver function. A scatter plot of the delay to the first report of a death or deaths against launch date showed that the more recent the launch date, the sooner deaths were reported (Figure 1). There was a similar relationship between the time to first withdrawal in any country and the launch year (Figure 2). This could be because of better reporting of suspected adverse reactions, better methods of assigning causality, or stricter regulation. Fig. 1: Time to first reported death versus launch year 

1. Aronson JK. Drug withdrawals because of adverse effects. In: Side Effects of Drugs, Annual 30. Amsterdam: Elsevier, 2008: xxxi-xxxv.
|


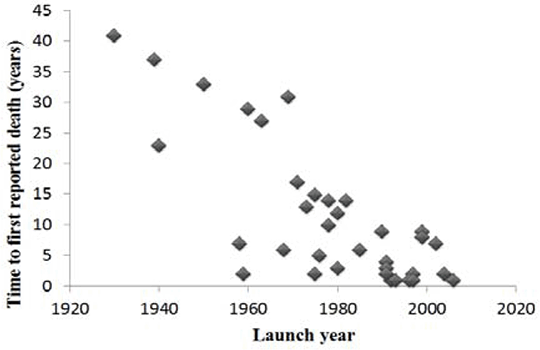 Fig. 2: Time to first withdrawal versus launch year
Fig. 2: Time to first withdrawal versus launch year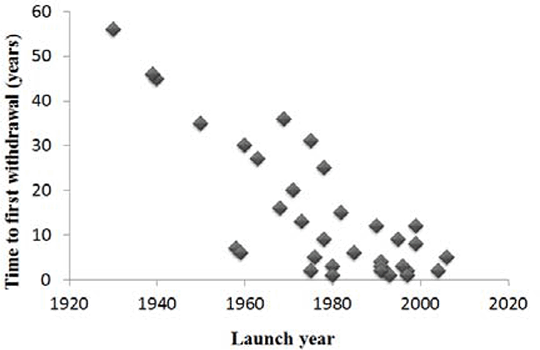 Reference
Reference