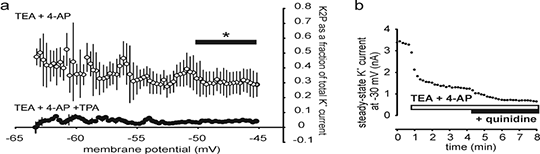
Function of voltage-insensitive, background K+ leakage in primary sensory neurons, and its block by tetrapentylammonium (TPA) ions and quinidine A heritable predisposition to migraine headache is associated with a loss of function mutation in TRESK (KCNK18) (1), suggesting that two-pore domain K+ channels (K2P) in human trigeminal and dorsal root ganglion neurons play a role in controlling neuronal excitability in pain states. However, attempts to functionally isolate the voltage-insensitive, background K+ leakage, generated by K2P, have been hampered by a lack of reliable pharmacological tools and this has resulted in attempts to analyse neuronal ‘background’ K+ currents, that are probably composed of a variety of non or slowly –inactivating K+ currents (2). The aim of this study was to more fully detail the contribution of voltage-insensitive, background K+ channels to membrane currents in sensory neurons and to examine the possible use of intracellular TPA and extracellular quinidine as tools to reveal K2P function. Primary sensory neurons were isolated and cultured from the dorsal root ganglia of young adult male Wistar rats, using standard techniques (3). K2P channels are insensitive to extracellular 10 mM tetraethylammonium (TEA) and 500 μμM 4-aminopyridine (4-AP), and we used these agents to block voltage-dependent K+ currents, in order to study the characteristics of the remaining minor component of outward-rectification in whole-cell, patch-clamp recordings. When TPA (1 μM) was added to the pipette solution, the residual background component was also abolished (Fig 1a). 
Fig 1 a Fraction of total K+ current appearing as background in small diameter DRG neurons, after block with TEA + 4-AP and intracellular TPA, versus membrane potential (means ± sem, n = 5,6, p <0.05, t-test). b 100 μM quinidine blocks K+ leakage from the outside by 52.7 ± 8.0% (means ± sem, n = 4). Our results indicate that intracellular TPA can block K2P selectively. Extracellular application of 100 μM quinidine following TEA and 4-AP partially inhibits K2P (Fig 1b), although observations on changes in membrane excitability using quinidine as a K+ channel blocker are unreliable as it also acts on Na+ channels. We suggest K2P channels may contribute to the control of excitability in neurons relying on low-threshold tetrodotoxin-sensitive Na+ channels for electrogenesis (including A-fibres and CGRP containing peptidergic neurons), rather than those nociceptors primarily utilizing NaV1.8. (1) Lafrenière RG et al Nat Med 16:1157, 2010 (2) Dobler T et al J Physiol 585:867, 2007 (3) Snape A et al J Physiol 588:125, 2010
|