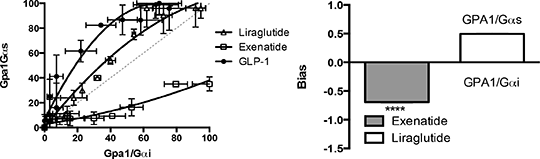
Investigating The Signalling Bias Engendered By GLP-1 Peptide Mimetics Glucagon-like-peptide 1 (GLP-1) acts via a 7 transmembrane receptor that couples, predominantly, to the Gαs subunit stimulating insulin release when blood glucose is elevated. In recent years, treatment of obese or diabetic patients with GLP-1, or one of its longer lasting analogues has been of significant pharmaceutical interest; increasing weight loss and combating many diabetic symptoms [1]. Despite several successful drugs approved for clinical use, our understanding of the underlying mechanisms for their effects remain poorly characterised. This is particularly significant given the observed clinical differences between highly similar mimetics and possible cancer risks associated with prolonged use. The GLP-1R is known to couple to multiple G protein subunits, including both the stimulatory Gαs and inhibitory Gαi families. Current studies predominantly uses cAMP (a product of both these pathways) as a read-out for GLP-1R activation. In this study we sought to use a system in which to isolate the individual contribution of the G protein subunits and investigate the potential for ligand-bias when using the different mimetics. We expressed the human receptor in the yeast system developed by GSK [2] expressing individual Gα chimeras adapted to enable coupling of mammalian GPCRs to the endogenous yeast-mating pathway. This pathway has been modified to include a reporter gene enabling characterisation of ligand-GPCR-G protein pharmacology in isolation. We stimulated the GLP-1R with the natural peptide agonist, GLP-1 (GLP-1(7-36)amide) or one of its clinically used mimetics (Liraglutide and Exenatide) to compare the effect of the G protein chimera on signalling. We report, for the first time, functional expression of the human GLP-1R in the yeast system and demonstrate coupling to several Gα subunits. The natural ligand activates the receptor-Gαs coupling with a pEC50 = 8.1±0.1 however, a reduced potency (pEC50 = 7.5±0.1) is observed when coupled to Gαi. The receptor is similarly expressed and trafficked to the membrane in both yeast strains and the antagonist, Exendin-9 inhibits both responses with pIC50 = 8.1±0.1 using 1x10-7 M agonist concentration suggesting that the differences in potency result directly from the Gα subunit present. 
Figure 1: Determining ligand-bias at the GLP-1R. Equimolar and equiactive bias comparisons of GLP-1R agonist activation of Gαs and Gαi calculated as outlined in [3]. Liraglutide was a full agonist with similar potency to the natural ligand on both pathways (pEC50 = 7.5 ±0.06 and 7.1 ±0.06). Exenatide was a partial agonist o the GLP-1R-Gαs coupling (log K A = -6.2 ±0.3, log τ = 0.06±0.01) displaying significant (p<0.0001, n=5; ANOVA) bias towards the inhibitory pathway relative to both GLP-1 and Liraglutide (Figure 1). This study highlights the pharmacological consequences of functional selectivity and establishes a system for further analysis of ligand-GLP-1R-Gα specificity. 1. Meier, J. J. (2012) Nat. Rev. Endocrinol. 8: 728-742. 2. Brown, A. J. et al (2000) Yeast 16:11-22. 3. Rajapol, S. et al (2011) Mol. Pharmacol. 80: 367-377.
|