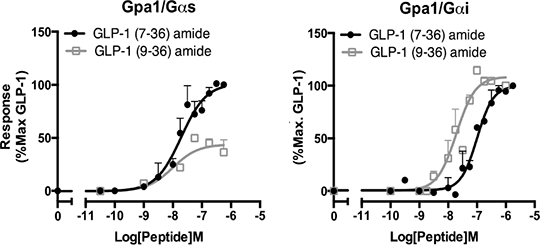
Investigating The Regulation Of GLP-1R Signalling By GLP-1(9-36)amide Glucagon-like peptide-1 (GLP-1) is released in the pancreas following food ingestion. Tissue specific processing results in the production of the active GLP-1(7-36)amide (hereafter GLP-1) form, which activates the GLP-1 receptor (GLP-1R) to mediate its incretin affects, predominantly through elevation of cAMP (governed by a balance of stimulatory and inhibitory G protein pathways). Given its central role in controlling postprandial blood sugar levels, the GLP-1R has become a major target for the management of diabetes and obesity with several mimetics of GLP-1 clinically approved. GLP-1 has a plasma half-life of only a few minutes being rapidly degraded by the serine-protease DPPIV to GLP-1(9-36)amide [1]. There are several conflicting reports on the activity of GLP-1(9-36)amide being described as inactive [1], a partial agonist [2] and an antagonist of GLP-1 [3]. Given that GLP-1(9-36)amide is present at a concentration five to ten fold higher than GLP-1 we sought to investigate its effect on the GLP-1R response to the clinically used mimetics (Liraglutide and Exenatide) in particular with respect to the effect of the different, opposing, G protein couplings known to exist for the GLP-1R. In this study we expressed the human GLP-1 receptor in a yeast system developed by GSK [4] containing individual Gα chimeras representing the stimulatory (Gαs) and inhibitory (Gαi) families, adapted to enable coupling of mammalian GPCRs to the endogenous yeast-mating pathway. This pathway has been modified to include a reporter gene enabling characterisation of ligand-GPCR-G protein pharmacology in isolation from competing pathways. We stimulated the GLP-1R with the natural peptide agonist, GLP-1 and its mimetics in combination with GLP-1(9-36)amide to compare the effect of the G protein coupling on the modulation of the signalling response. GLP-1(9-36)amide was a partial agonist of the GLP-1R-Gαs response (log KA = -7.4 ±0.1 and log τ = -0.14±0.01) but a full, more potent agonist of Gαi (Figure 1). Significantly, the breakdown product acted as an antagonist of GLP-1 at Gαs. However, when interaction experiments were performed in the presence of the Gαi subunit, GLP-1(9-36)amide potentiated the response. Interestingly, GLP-1(9-36)amide displayed different effects when the GLP-1 mimetics were used to stimulate a response with weaker inhibition of Gαs response and little potentiation observed for Gαi signalling. These data provide further insight into the molecular mechanisms used by the peptide drugs. Importantly, these data characterise the effect of GLP-1(9-36)amide on the GLP-1R response to the clinically used mimetics. Understanding these differences are particularly significant given the clinical differences observed between the mimetics and the reported increased cancer risks associated with prolonged use. 
Figure 1: Determining the effect of G protein subunit on GLP-1(9-36)amide pharmacology 1. Metlein, R. et al. (1993) Eur. J. Biochem. 214: 829-835. 2. Montrose-Rafizadeh, C. et al. (1997) J. Biol. Chem. 272: 21201-21206. 3. Knudsen, L., B. et al., (1996) Eur. J. Pharmacol. 318: 429-435. 4. Brown, A. J. et al (2000) Yeast 16:11-22
|