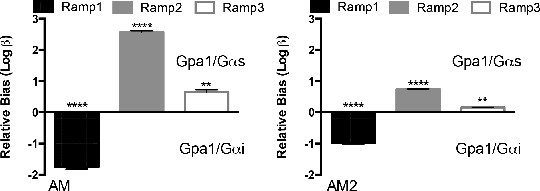
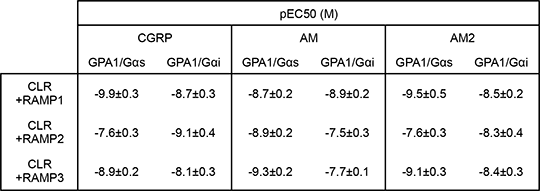
Exploring The Effect Of The G Protein Subunit On RAMP-Mediated Signal Modulation Of Calcitonin-related-like Receptor Receptor-activity modifying proteins (RAMPs) have been shown to interact with a number of GPCRs including the calcitonin-related-like receptor (CLR) [1]. Interaction with each of the three identified RAMPs modulates receptor signal transduction in a ligand-dependent manner effectively producing three receptors with distinct pharmacology. Three related peptide ligands, calcitonin gene-related peptide (CGRP), adrenomedullin (AM1) and adrenomedullin 2 (AM2, also termed intermedin) interact with the CLR. Binding and receptor activation (measured as cAMP accumulation through activation of Gαs) are determined by the presence of each RAMP. The CLR has also been shown to couple to the inhibitory Gαi subunit [2] however, little is known about the affect of RAMP and ligand-induced signal bias with respect to the two competing G protein subunits for this receptor. We expressed the CLR in combination with each of the RAMPs in the GSK yeast system [3] containing either the Gαs or Gαi chimera adapted to enable coupling of receptor activation to the endogenous yeast-mating pathway modified to include a reporter gene. Reporter gene activity was measured following 17 h stimulation with ligand. Data is plotted as mean of at least 5 independent experiments and pEC50 values determined through fitting of a 3-parameter concentration-response curve. Bias factors (log β) were calculated as detailed in [4] with respect to CGRP agonism. Ligands were considered bias if log β was significantly different from 0 (p<0.05). Co-expression of RAMP1 with CLR resulted in the formation of a CGRP receptor in presence of the Gαs subunit having broadly similar pharmacological properties to those observed for mammalian cAMP assays (potency of CGRP=AM2>AM). Similarly RAMP2 expression reconstituted an adrenomedullin receptor (AM>CGRP=AM2). For RAMP3 the rank potency order was that of the adrenomedullin-2 receptor (AM>AM2>CGRP) but there was loss of discrimination. Significantly, when the same combinations of RAMP and CLR were expressed in the yeast strain containing the Gαi chimera differing potency rankings were observed (Table 1) suggesting ability for ligands to exhibit a RAMP and G protein signal bias as shown in the equiactive bias plot (Figure 1). This study offers an interesting and novel insight into the effect of G protein subunit on RAMP-mediated modulation of signal transduction. 
Figure 1: Relative (to CGRP) ligand-bias for the activation of Gas and Gai calculated as outlined in [4]. Statistical significance determined as ****p<0.0001 and ** p<0.01. 
Table 1: pEC50 values for CLR peptide ligands 1. McLatchie, L., M. et al. (1998) Nature 393: 333-339. 2. Zhang, X-H., et al. (2007) Am. J. Physiol. 293: H2888-H2893. 3. Brown, A. J. et al (2000) Yeast 16:11-22. 4. Rajapol, S. et al (2011) Mol. Pharmacol. 80: 367-377.
|