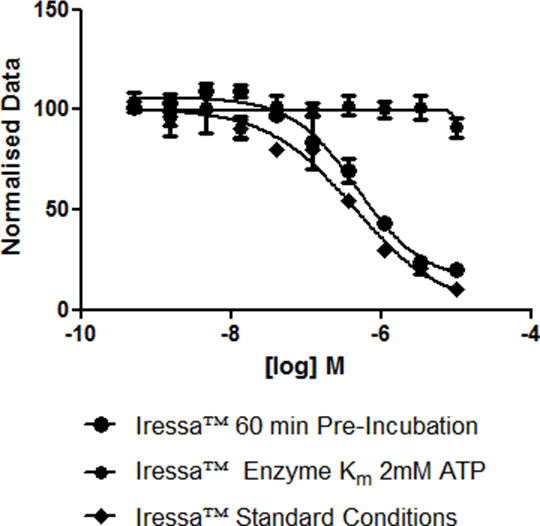
HighThroughput Enzymology Studies of EGFR Inhibitors with Differing Mechanisms. High Throughput Screening (HTS) is a well established paradigm for hit identification in pharmaceutical R&D. With compound libraries running into millions of discrete structures, the key advantage of HTS is the ability to cover diverse chemical space in a single, well validated and quality controlled process. Whilst the HTS paradigm offers a key advantage by using maximum throughput to achieve results in a shortened time period, it classically has not been able to deliver fully characterised hits where mechanism of target interaction is unknown whilst maintaining the throughput that drug-discovery programs have become accustomed to. This presentation discusses the use of a decision tree that builds upon traditional high throughput diversity screening, with the aim of maximising mechanistic information. Exemplified by a full collection HTS campaign against EGFR running assays at K M and physiologically relevant concentrations of substrate together with compound pre-incubation, I will highlight differences in compound profile and relate this to differing compound mechanism of action [1]. Primary screening utilising a commercially available HTRF assay resulted in selection of 5400 compounds for concentration response confirmation based on an activity criterion of greater than 50% kinase inhibition. The selected compound set was subjected to two further biochemical studies by modification of the existing assay format. An observed pIC50 shift of greater than 0.5 of a log order was considered an indicator of a mechanistic difference. Further to these studies the LanthaScreen™ Eu Kinase binding assay was utilised to determine the off rate for known kinase inhibitors that had previously been examined in the high throughput annotation assays. Figure 1 -Iressa™ Test Conditions
The resulting data showed 15.2% of the compounds tested increased in potency with an extended incubation of Enzyme with compound. The KM and physiological ATP assay showed a potency shift in 7.5% of the tested collection. Iressa™ (Figure 1) was shown to have no shift in potency with differing pre-incubation conditions. This observation was further confirmed by an off rate of 9 minutes. Mechanistic profiling of compounds within pharmaceutical R& D is traditionally a Lead Optimisation activity, focused on smaller numbers of compounds. This will continue to be necessary for a detailed understanding of mechanism of action, however I would propose that the decision tree described in this presentation should be used, where possible, to highlight any mechanistic differences between hit compounds, thus aiding triage of large compound numbers and focusing chemistry efforts on chemical series with the desired mechanistic profile. 
1. Wood ER, Truesdale AT, McDonald OB et al. (2004) A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): Cancer Res 64(18):6652–6659.
|