Print version
Search Pub Med
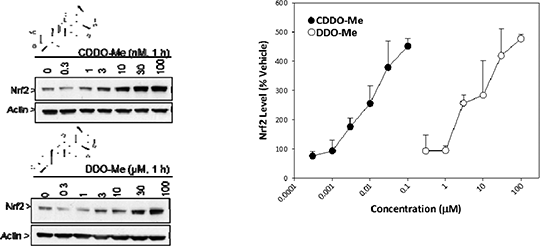
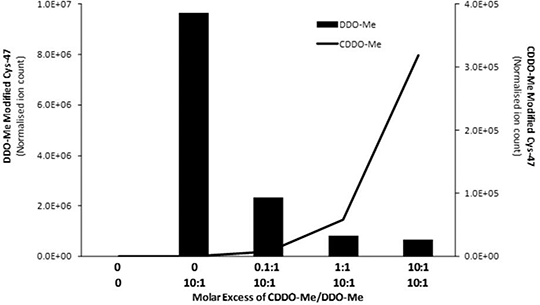
Towards the Chemical Mechanism of Action of CDDO-Me - a Potent Inducer of the Nrf2 Cell Defence Pathway The Keap1-Nrf2 pathway protects mammalian cells against the deleterious effects of chemical and oxidative stress. The semi-synthetic triterpenoid CDDO-Me (methyl-2-cyano 3,12-dioxooleano-1,9- dien-28-oate; also known as bardoxolone methyl) is one of the most potent pharmacological activators of Nrf2, yet the chemical and molecular basis of its activity is poorly understood. Knowledge of these facets would considerably enhance our ability to design therapeutic agents capable of manipulating the cytoprotective activities of Nrf2 for the benefit of human health. Therefore, the aim of this study was to delineate the chemical mechanism(s) underlying the extraordinary potency of CDDO-Me as an inducer of Nrf2 signalling, and to specifically test the hypothesis that CDDO-Me modifies specific cysteine residues in Keap1, the redox-sensitive cytosolic repressor of Nrf2. Published data indicates that CDDO-Me is capable of reacting with thiols in a reversible manner, and we rationalised that this would severely hinder attempts to characterise the ability of the compound to modify Keap1 using liquid chromatography mass spectrometry (LC-MS). Therefore, we synthesised the novel analogue DDO-Me, which lacks a cyano moiety adjacent to the A-ring enone, and used nuclear magnetic resonance to demonstrate its ability to form a stable adduct with β-mercaptoethanol in vitro. We also determined, by immunoblotting, that DDO-Me is three orders of magnitude less potent than CDDO-Me as an inducer of Nrf2 in murine Hepa-1c1c7 cells (Fig. 1). Using the model protein glutathione S-transferase P1 (GSTP1), in which Cys-47 is highly reactive towards electrophiles, we have developed an LC-MS assay enabling the direct detection, for the first time, of CDDO-Me/DDO-Me adducts on a protein. Using this approach, in which purified recombinant GSTP1-His is exposed to increasing molar excesses of DDO-Me (24 h) or CDDO-Me (1 h) at room temperature, before LC-MS analysis of peptides yielded by trypsin digestion of the protein, we provide evidence that DDO-Me is capable of forming a stable adduct with GSTP1, and that CDDO-Me can competitively inhibit this modification (Fig. 2). We are currently employing DDO-Me as a chemical tool to probe the ability of this class of compound to directly modify Keap1, and to unequivocally identify the site(s) of modification. These efforts are supported by our use of molecular modelling to predict candidate sites of modification in Keap1. This work will provide insights into the chemico-biological traits that underlie the potency, and relative safety, of CDDO-Me and related compounds as inducers of Nrf2 signalling. 
Figure 1: Immunoblotting for total cellular Nrf2 shows differential potencies of CDDO-Me and DDO-Me as inducers of Nrf2 
Figure 2: Modification of Cys-47 of GSTP1 by DDO-Me, and concentration-dependent competitive inhibition by CDDO-Me |