Print version
Search Pub Med
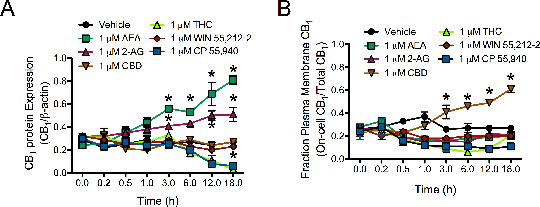
Different Type 1 Cannabinoid Receptor Ligands Either Enhance ERK Signaling Or Promote Arrestin2-Mediated Receptor Desensitization In Cultured Cells That Model Striatal Medium Spiny Projection Neurons Modulation of type 1 cannabinoid receptor (CB1) activity has been touted as a potential means of treating addiction, anxiety, depression, Huntington’s disease, and other neurological disorders. As agonists of CB1 differ in their chemical structure and in vivo efficacy, we hypothesized that different classes of cannabinoids exert different effects on CB1 receptor trafficking and intracellular signalling. We used bioluminescence resonance energy transfer (BRET)1, In-cell™ western assays2, and qRT-PCR2 to examine the functional selectivity of 6 representative cannabinoids that are widely used to study CB1: 2 endocannabinoids [2-arachidonyl glycerol (2-AG) and anandamide (AEA)], 2 synthetic cannabinoids (WIN 55,212-2 and CP 55,940), and 2 phytocannabinoids [cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC)] (all in DMSO vehicle). CB1-dependent Gi/o-, Gs-, and arrestin2-mediated intracellular signaling were examined in the mouse STHdh cell culture model of striatal medium spiny projection neurons. Endogenous expression of CB1 is approximately 10-fold higher than expression of the type 2 cannabinoid receptor (CB2) in STHdh cells. We observed that 2-AG and AEA treatment biased CB1 signaling toward Gi/o-dependent activation of ERK [2-AG (76±4%); AEA (80±10%); vehicle (20±2%)] and increased total CB1 expression (Fig. 1A). CBD treatment enhanced Gs-dependent CREB activation [CBD (79±7%); vehicle (34±3%)] and increased plasma membrane CB1 abundance, but did not affect total CB1 levels (Fig. 1A,B). THC and CP 55,940 treatment increased arrestin2-dependent ERK phosphorylation [THC (82±5%); CP 55,940 (64±7%); THC and over-expression of a dominant-negative arrestin2 (21±4%); vehicle (20±2%)] and enhanced CB1 internalization and receptor down-regulation (Fig. 1A,B). Therefore, treatment with 2-AG and AEA increased the expression of CB1 whereas treatment with THC or THC-like compounds (CP 55,940) reduced the CB1 levels in our cell culture model. 
Fig. 1 Effect of cannabinoids on total CB1 protein expression (A) and CB1 abundance at the plasma membrane (B). *p<0.05 vs. vehicle within time point two-way ANOVA and Bonferroni’s post-hoc test, n ≥ 4. In summary, therapies designed to enhance or maintain CB1 signaling should exploit the functional selectivity of endocannabinoids while avoiding the internalization and degradation of CB1 observed here when cells were treated with THC. In the case of Huntington’s disease, increasing endocannabinoid tone may restore CB1 signaling, whereas THC-like compounds may exacerbate CB1 loss. References 1) James et al, Nat Methods 3:1001-6, 2006 2) Laprairie et al, Neuropharmacol 72:47-57, 2013
|