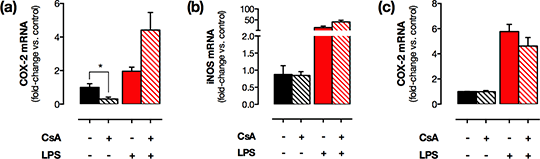
Distinct transcriptional regulation of constitutive versus inflammatory Cox2 gene expression: implications for novel COX-2-targetted therapies that spare the cardiovascular system Non-steroidal anti-inflammatory drugs such as diclofenac and celecoxib inhibit cyclo-oxygenase (COX)-2 to produce anti-inflammatory and analgesic benefit, but these are associated with renal and cardiovascular toxicity. In contrast to the established view, we have recently shown that in health COX-2 is absent in vessels but highly expressed in discrete regions of the brain, gastrointestinal tract, thymus and, of particular relevance to cardiovascular side effects, the kidney. COX-2 is rapidly induced in most tissues by cytokines and mitogens through NFκB and MAPK pathways but the mechanisms regulating constitutive COX-2 expression are not known. Previous reports have shown renal COX-2 is suppressed by calcineurin inhibitors such as cyclosporine A (CsA), suggesting that, in the kidney, constitutive COX-2 expression is driven by the transcription factor, NFAT. Here we set out to compare the effect of inhibition of the calcineurin/NFAT pathway with CsA on constitutive COX-2 expression in the kidney vs other expressing tissues, and COX-2 induced at these sites during inflammation. C57Bl/6 mice (n=4-6/ group, male, 10 wks old) were treated with CsA (15mg/kg/day; s.c.) or vehicle (4% ethanol, 6% Cremaphor-EL, 90% saline) for 4 days. Mice then received LPS (10mg/kg; i.p.) or vehicle (saline) and were culled 4hrs later by CO2 narcosis. Renal medulla, brain, thymus and spleen were removed and RNA extracted. COX-2, COX-1 and iNOS mRNA levels were quantified by qRT-PCR. Data were normalised to housekeeping genes (GAPDH+18S) and quantified by the comparative Ct method. COX-2 was detected in all tissues with the rank order brain>renal medulla>>thymus>>spleen. CsA treatment reduced constitutive COX-2 expression in the renal medulla (Figure 1a) but not expression of COX-1 (1.3±0.2-fold; p=0.47) or iNOS (Figure 1b) indicating a specific effect on COX-2 pathways. By contrast, CsA had no effect on levels of COX-2 in the brain (Figure 1c), thymus or spleen. LPS tended to increase COX-2 and iNOS expression in all studied tissues with the strongest effect observed in the spleen (COX-2: 61±14–fold, p<0.01; iNOS: 13.8±6.4-fold, p=0.06) but did not alter COX-1 expression. In LPS-treated mice, CsA had no effect on COX-2, iNOS or COX-1 expression levels in any tissue. 
Figure 1 CsA treatment reduced constitutive but not LPS-induced COX-2 levels in the renal medulla (a) but had no effect on renal iNOS (b) or brain COX-2 levels (c). *; p<0.05 by unpaired t-test. These data suggest that COX-2 in the kidney is regulated by distinct transcriptional pathways to COX-2 at other sites and the induction of COX-2 by inflammatory stimuli. Importantly, this implies that inflammatory COX-2 induction could be therapeutically targeted at the transcriptional level to produce an anti-inflammatory benefit, without influencing the constitutive COX-2 activity necessary for renal/cardiovascular homeostasis.
|