Print version
Search Pub Med
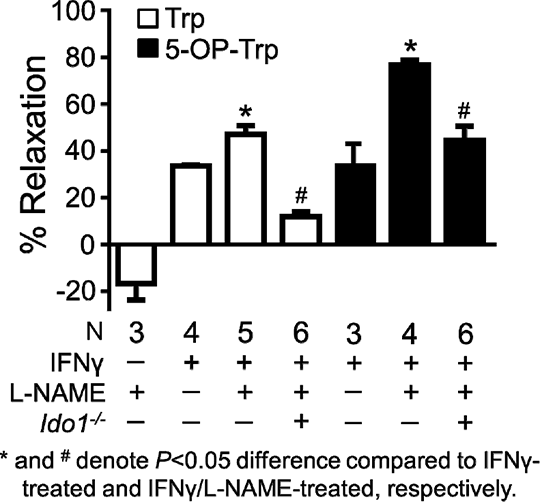
Contribution Of Indoleamine 2,3-dioxygenase-1 To The Regulation Of Arterial Relaxation The pro-inflammatory cytokine interferon-γ (IFNγ) activates the heme enzyme indoleamine 2,3-dioxygenase-1 (IDO1) to initiate the metabolism of the essential amino acid L-tryptophan (Trp) along the L-kynurenine (Kyn) pathway. Recent work has shown that in a mouse model of endotoxaemia, Trp metabolism by IDO1 mediates endothelium-dependent relaxation, contributing to hypotension (1). The aim of this study was to characterise tryptophan-mediated relaxation in the mouse abdominal aorta, and to chemically identify Trp-derivatives responsible for arterial relaxation under inflammatory conditions. Adult C57BL/6J mice were injected intraperitoneally with 7.5 mg/kg lipopolysaccharide (LPS) and their abdominal aortae removed 16 h later. Alternatively, aortae isolated from C57BL/6J or Ido1 –/– mice were treated with IFNγ (100 ng/mL) for 48 h in M-199 medium. Aortic rings were mounted in a wire myograph and placed under a basal tension of 9 mN. Vessels were initially constricted maximally with 60 mM KCl and subsequently pre-constricted to approximately 50% of maximum with norepinephrine. Responses to Trp, its analogue 5-O-propyne tryptophan (5-OP-Trp; synthesised de novo) and Kyn were determined in the presence of various inhibitors. Also, the products formed from Trp and 5-OP-Trp by IDO1-expressing endothelial (EC) and smooth muscle cells (SMC) were determined by liquid chromatography-mass spectrometry (unpublished method). Statistical differences were assessed by Student’s t test. Trp (8 mM) relaxed aortae isolated from LPS-treated mice by 34±4% (n=15) which was attenuated by the IDO inhibitor 1-methyl tryptophan (1-MT; 1 mM; 12±4%, n=6). Trp relaxed aortic rings pre-treated with IFNγ in vitro, but not naïve vessels (Fig.1). 5-OP-Trp (8 mM) produced relaxations similar to Trp, both of which were enhanced significantly in vessels treated with the nitric oxide synthase inhibitor L-NAME (100 μM; Fig. 1). Trp and 5-OP-Trp were converted to Kyn and 5-OP-Kyn, respectively, in IDO1-expressing EC and SMC. Trp-induced relaxation was increased by O-(carboxymethyl) hydroxylamine, an inhibitor of Kyn aminotransferases (KAT). Relaxation to Trp and 5-OP-Trp was decreased in aortae from Ido1 –/– mice (Fig. 1). Interestingly and contrary to previous observations with commercial Kyn (1), HPLC-purified Kyn did not cause immediate relaxation, although it ‘gained’ vessel relaxation activity upon pre-incubation at 37°C. 
In summary, IDO1-expressing mouse abdominal aortae relax in response to Trp. This relaxation appears to be regulated by nitric oxide, which has been shown previously to modulate the activity of IDO1 (2). Furthermore, Trp metabolism to kynurenic acid by KAT seems to compete for formation of the Trp-derived relaxant. Our findings with HPLC-purified Kyn suggest that a Trp-derivative rather than Kyn itself is responsible for relaxation. Studies utilising Trp analogues such as 5-OP-Trp in conjunction with click chemistry are currently under way to identify the endogenous Trp-derived relaxant. (1) Wang Y et al. Nat Med 16:279, 2010 (2) Thomas SR et al. J Biol Chem 269:14457, 1994
|