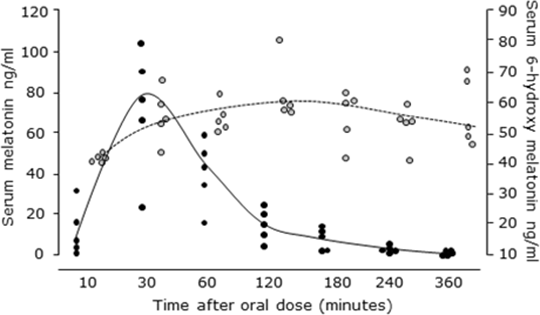
Evaluation of High Dose Melatonin in Healthy Volunteers to Inform a Phase II Trial in Patients with Sepsis Melatonin accumulates in mitochondria and both it and its metabolites have potent antioxidant and anti-inflammatory activity, preventing organ dysfunction in a rat model of sepsis. We propose that melatonin may be useful in sepsis but limited data exists as to the required dose and dosing interval. We undertook a Phase I open label dose escalation study in healthy volunteers to assess the tolerability and pharmacokinetics of exogenous melatonin and its major metabolite and related these doses to ex vivo anti-inflammatory and antioxidant activities in a whole blood model of sepsis. The study was approved by the Scotland A Research Ethics Committee and the Medicines and Healthcare Regulatory Authority and all subjects gave written informed consent. Twenty healthy, non-smoking male subjects (aged 18-30) were recruited. Each volunteer received a single dose of oral melatonin, with 5 subjects in each dose cohort (20, 30, 50 and 100mg). Subjects fasted overnight and had basal measures of heart rate, blood pressure, and oxygen saturation and an ECG was performed. A venous cannula was inserted for blood sampling for routine biochemistry and full blood count. Subjects then took a single oral dose of melatonin as 10mg capsules and blood samples were collected at intervals for 6h, then again at 24h, for measurement of melatonin and its main metabolite. Any symptoms were recorded and physiological measures were performed at 30min intervals. Urine was also collected. Melatonin and its major hydroxylation metabolite, 6-hydroxymelatonin (measured as 6-hydroxymelatonin sulphate) were measured using HLPC and enzyme immunoassay respectively. No adverse effects other than mild and transient drowsiness and no effects on sleeping patterns were seen and there were no symptoms reported at any dose. All physiological and biochemical measures were normal at all doses. Melatonin was rapidly cleared with similar elimination half lives at all doses. There was considerable variability in the Cmax melatonin levels within each dose cohort. The individual results for the 50mg dose are shown in Fig 1 (solid circles). Concentrations of 6-hydoxymelatonin were less variable and remained stable for several hours (Fig 1, open circles), and were similar at both 50mg and 100mg doses of melatonin. Urine 6-hydroxymelatonin excretion over 6h was broadly dose related (3,7, 5.1, 14.0 and 37.1mg at doses of 20mg, 30mg, 50mg and 100mg respectively (median values, n=5 per dose cohort). We have shown that an oral melatonin dose of 50mg will achieve median Cmax levels of ~75ng/ml melatonin resulting in median Cmax levels of 6-hydroxymelatonin of around 50-70ng/ml which we have shown are effective at reducing inflammation and oxidative stress under conditions mimicking sepsis in an ex vivo whole blood model [1]. We will now proceed to a Phase II study in patients with sepsis. 
1. Lowes et al. Br J Anaesth 111:309P, 2013. Funded by the Chief Scientist Office of Scotland. |