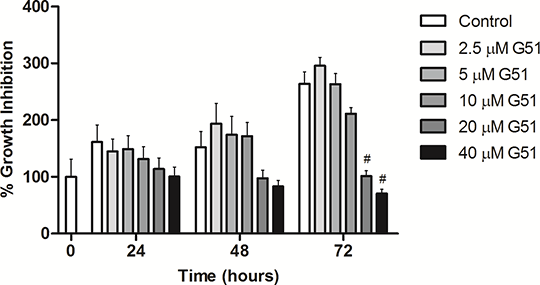
Parameterising PDK1-Induced Perturbations on the Dose-Response Profile of Human Glioblastoma Twenty first century drug discovery & development welcomes a shift in its scientific background. Despite the innovative developments during the last decades, this process is still inefficient. The main objectives for 2020 on this matter are clearly focused in improving the whole process (1)(2). In order to achieve this goal, several strategies have been pursued, namely the novel concept of Systems Pharmacology (3). The present study aims to evaluate the perturbations induced by the PDK1 inhibitor, G51 (C25H25N5O4), on the proliferation and viability of a human glioblastoma cell line (U-87 MG), applying the concept of Systems Pharmacology to drug development. This can be critical for the treatment of Glioblastoma Multiforme (GBM), which remains therapeutically challenging (4). In order to evaluate the induced perturbation by the inhibitor in terms of cell growth, the amount of sulphorhodamine B (SRB) bound to the cell proteins was measured as described elsewhere (5). A range of 5 doses from 2.5 µM to 40 µM was tested for incubation times up to 72h. The results were quantified through visible spectrometry at 540 nm. Statistical treatment of the data was carried out using GraphPad Prism 5.00 and the One-Way ANOVA method, followed by multiple comparison Turkey’s test. This preliminary drug screening shows a significant perturbation of the cell line after 72h of treatment with G51 at 20 µM and 40 µM concentrations. Additionally, the mean 50% growth inhibition concentration (GI50) were obtained for this compound at 24h, 48h and 72h: 9.26 µM, 14.72 µM and 10.96 µM respectively. 
Fig. 1 Perturbation effect in the growth of the cell line U-87 MG by the inhibitor G51. # p < 0.001 vs control. The results thus gathered stand as a proof of concept for a measurable cell perturbation by this PDK1 inhibitor, regarding its viability and cell-growth ability. The study will be complemented with further proteomic analysis, with a view to translate the cell perturbation data to the proteins network. This is particularly relevant since the successful treatment of diseases mandates that the newly developed drugs are able to influence the synthesis and metabolism of proteins with clinical importance (e.g. as biomarkers). (1) Csermely P et al, Elsevier Inc.138(3):333–408. (2) Arlington S, From vision to decision - Pharma 2020 1–52, 2012 (3) S Sorger PK et al, An NIH White Paper by the QSP Workshop Group 0–47, 2011 (4) Desouza L V et al, Molecular cancer 12(1):74, 2013 (5) Papazisis KT et al, J. Immunol. Methods 208(2):151–8, 1997 |