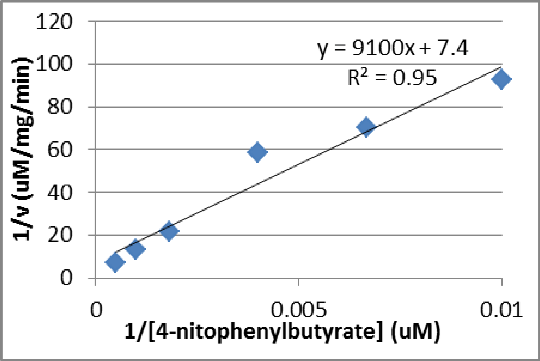
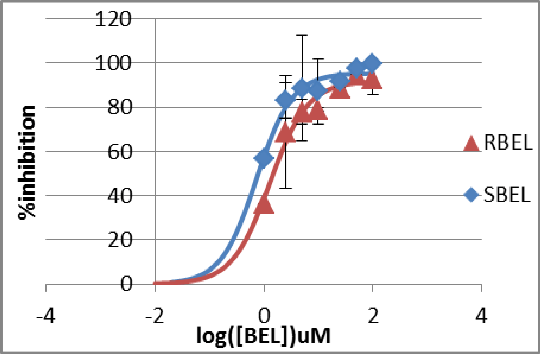
Purification and inhibition studies of the calcium independent phospholipase A2β orthologue patatin isolated from Solanum tuberosum Calcium independent phospholipase A2β (iPLA2β) is putatively involved with vascular inflammation,[1] hence identifying antagonists of the enzyme could lead to potential new therapeutic drugs in the fields of hypertension and cardiovascular disease. In order to establish an in vitro screen for inhibitors less toxic than bromoenol lactone (BEL), the archetypal 43 kDa serine hydrolase patatin isolated from potato tubers has been selected. Potato (175-250 g) was homogenised using established methods with tris HCl (25 mM, pH 7.4), polyclar AT (10 g) and 10 mM ascorbic acid. The mixture was clarified by centrifugation (3500 x g) and then at (10000 x g) before ion exchange chromatography using DEAE Sepharose. The eluate was passed through concanavalin A Sepharose to give highly purified enzyme with typical activity of 3.6 ± 2.3 min-1 uM-1 mg-1. Enzyme varied somewhat between preparations, but catalytic efficiency was always close to the published value of 4 min-1 uM-1 mg-1. The rate of 4-nitrophenylbutyrate (2 mM in 2% v/v aqueous DMSO working concentration) hydrolysis was recorded using a spectrophotometric assay at 410 nm, in the presence and absence of enantiomeric BEL ligands dissolved in water. Analysis showed that the assay followed classic Lineweaver-Burk kinetics (Fig.1). Optimal conditions for the antagonist screen were determined to be 2 mM 4-nitrophenyl butyrate at 25 oC using 20-25 μg enzyme per assay. R- and S-BEL were separately tested for antagonism (Fig. 2) both giving IC50 values in the μM range. Binding has been simulated using Autodock Vina and 1oxw.pdb followed by molecular dynamics with AMBER 12, which demonstrated each enantiomer can dock in the known active site. 
Figure 1. (left) Lineweaver-Burk plot for patatin. 
Figure 2. (right) A log dose response curve for percentage inhibition of initial rate caused by the presence of S-BEL and R-BEL Patatin was found to provide a straightforward assay for iPLA2β-like activity. Combining this with in silico studies of novel antagonists, the process has the potential to form the basis of a high-throughput screen for compounds of therapeutic value in cardiovascular medicine. 1. Lui S, Xie Z, Zhao Q et al. J Biol Chem. 287:24738, 2012 2. Pots. A M et al Eur. J. Biochem. 252:66, 1998
|