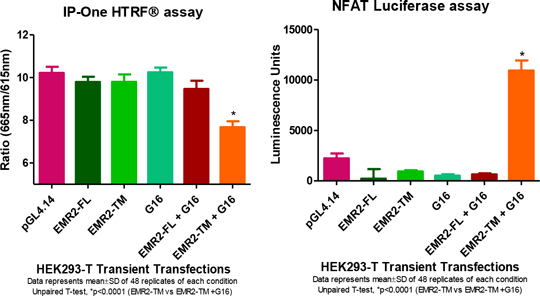
Demonstration of G-protein coupling of Adhesion GPCR EMR2 Adhesion G-Protein Coupled Receptors (aGPCRs), also known as Long N-terminal Family-B Receptors, are a group of ~30 proteins in the Family B class of GPCR superfamily. Adhesion-GPCRs are defined by their large extracellular N-termini region linked to a 7TM domain via a GPCR proteolytic site (GPS)-containing stalk region. The GPS is highly conserved in the EMR (EGF-like module-containing mucin-like hormone receptor-like) subfamily of aGPCRs containing tandem repeats of epidermal growth factor (EGF)-like at the N-terminus. Literature has shown autocatalytic cleavage at the GPS occurs in the endoplasmic reticulum compartment and the extracellular and 7TM domains are associated noncovalently by di-sulphide bridges. Separation of the N-Terminus from the 7TM domain is thought to activate the receptors constitutively. Prior to this study G-protein coupling had not been demonstrated for EMR2. We investigated the G-protein coupling pathways by co-expressing full length (EMR2-FL) and truncated transmembrane (EMR2-TM) domain of the EMR2 receptor with various G-proteins (Gα15, Gα16, Gα16z49) in mammalian cells and measured downstream intracellular molecules inositol phosphate and luciferase linked reporter gene transcription. The receptors were individually transiently co-transfected with a G-protein into HEK293 cells and incubated for 48hrs prior to addition of inositol phosphate and luciferase detection agents. The pGL4.14 plasmid was used as a negative control as it has the same backbone as NFAT plasmid minus the NFAT response element. Data represent mean±SD of fluorescent emission/excitation ratio for inositol phosphate assay and luminescence counts of luciferase assay and statistical significance determined by an unpaired T-test. Decrease in signal in the IP-One assay represents an increase in endogenous IP-1 production and indicates signal transduction via coupling to G16.

The data shows that truncation of EMR2 constitutively activated the receptor and signal transduction occurs via G16 coupling. This novel data confirms EMR2 is G-protein coupled and removal of the N-terminus activates the receptor.
|